Evaluation of a novel point-of-care lateral flow assay screening for Neisseria gonorrhoeae infection among pregnant women in Zimbabwe
- PMID: 39932971
- PMCID: PMC11813084
- DOI: 10.1371/journal.pgph.0003839
Evaluation of a novel point-of-care lateral flow assay screening for Neisseria gonorrhoeae infection among pregnant women in Zimbabwe
Abstract
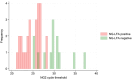
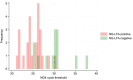
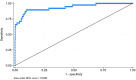
Affordable, easy-to-use and rapid diagnostics may support a move away from syndromic management for sexually transmitted infections (STIs) in resource-constrained settings. A lateral flow assay for Neisseria gonorrhoeae (NG-LFA) has shown high sensitivity and specificity (>90%) in symptomatic individuals. We investigated the performance and acceptability of this assay as a screening tool for NG among pregnant women. This evaluation was embedded within a prospective study evaluating point-of-care STI screening in pregnant women attending antenatal care (ANC) in Harare, Zimbabwe. Participants were included regardless of symptom status, ANC visit number, or gestational age. Nurse-collected vaginal swabs were tested on-site using the NG-LFA and the Xpert CT/NG assay (Xpert) (reference test). The implementation team members (n=4) were interviewed to assess acceptability and usability of NG-LFA. Of 912 participants, 4.8% (44/912) self-reported presence of abnormal vaginal discharge. Xpert NG prevalence was 4.2% (38/912); 81.6% (31/38) of infections were asymptomatic. The sensitivity, specificity, positive predictive value, and negative predictive value (NPV) of the NG-LFA were 65.8% (25/38; 95% CI 48.6%-80.4%), 99.2% (867/874; 95% CI 98.4-99.7%), 78.1% (25/32; 95% CI 60.0-90.7%), and 98.5% (867/880; 95% CI 97.5-99.2%). The NG-LFA was considered easy-to-use and interpret but discordant results led to issues of trust in the NG-LFA results. Among predominantly asymptomatic pregnant women, the NG-LFA had high specificity, but relatively low sensitivity meaning one in three cases of gonorrhoea were not detected. Further studies are warranted to assess the clinical performance and cost-effectiveness of the NG-LFA in other settings and populations.
Copyright: © 2025 Martin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
LTM is employed as a consultant by FIND. BB, BG and CF are employees of FIND. All other authors declare no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The prototype lateral flow assay and reader evaluated in this study were developed by FIND, in collaboration with the contract research organization DCN Dx, to facilitate gonorrhoea/chlamydia testing at the point of care in low- and middle-income countries. FIND aims to transfer the technology under a licensing agreement with Global Access terms to a commercial manufacturer. FIND is a global nonprofit connecting countries and communities, funders, decision makers, health care providers, and developers. FIND will not receive royalties from the sales of the final assay.
Figures
References
-
- World Health Organization. Guidelines for the management of symptomatic sexually transmitted infections. 2021. - PubMed
-
- Chaponda EB, Bruce J, Michelo C, Chandramohan D, Chico RM. Assessment of syndromic management of curable sexually transmitted and reproductive tract infections among pregnant women: an observational cross-sectional study. BMC Pregnancy Childbirth. 2021;21(1):98. doi: 10.1186/s12884-021-03573-3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
