Assessing the Risk of Relapse After In Vitro Fertilization in Women With Multiple Sclerosis
- PMID: 39933125
- PMCID: PMC11820809
- DOI: 10.1212/NXI.0000000000200371
Assessing the Risk of Relapse After In Vitro Fertilization in Women With Multiple Sclerosis
Abstract
Background and objectives: Older studies reported an increased risk of relapse after in vitro fertilization (IVF) in women with multiple sclerosis (MS), which has not been confirmed by more recent works. All these studies had several limitations, such as small sample sizes, absence of a control population, or lack of neurologic validation of the relapses. The aim of this study was to determine the risk of relapse after IVF in women with MS.
Methods: This retrospective cohort study included all women with MS who underwent IVF between 2009 and 2019 and a control group of women with MS who did not undergo IVF matched on age, MS duration, number of relapses, and MS-specific treatments in the previous year. Data on MS (disease duration, treatments, and relapses) were from the French MS Registry (OFSEP), whereas data on IVF (number of procedures, stimulation protocol type, and outcomes) were from the French national health insurance database. For this, the 2 databases were linked by indirect matching.
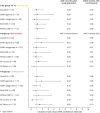
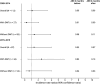
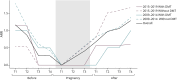
Results: In total, 115 women with MS underwent 199 IVF procedures (mean age at first IVF: 33.9 ± 4.0 years; 45.2% had ≥2 IVF procedures), and 175 IVFs (88.0%) could be matched to specific patients. The risk of relapse in the 3 months after index date was the same in both IVF group and control group (0.06 relapse per patient-year), as confirmed also by the before-after analysis in the IVF group (0.06 vs 0.08).
Discussion: This study, using a 10-year clinical and administrative dataset, did not find any increased risk of relapse after IVF. The maintenance of disease-modifying therapies until IVF was a determining factor in reducing the risk of relapse.
Conflict of interest statement
M. Mainguy and R. Casey report no disclosures relevant to the manuscript; S. Vukusic received grants, non-personal consulting fees, and travel fees from Biogen, Janssen, Merck, Novartis, Roche, Sandoz, and Sanofi; C. Lebrun-Frenay reports no disclosures relevant to the manuscript; E. Berger received honoraria and consulting fees from Novartis, Sanofi Aventis, Biogen, Genzyme, Roche, and Teva Pharma; A. Kerbrat reports no disclosures relevant to the manuscript; A. Al-Khedr has received consulting and lecturing fees, from Biogen, Novartis, Roche, Merck, Sanofi, Alexion, and Sandoz, travel grants from Novartis, Merck, and Alexion; B. Bourre has served on scientific advisory board for Alexion, BMS, Biogen, Sanofi, Janssen, Merck, Horizon, Novartis, Roche, and Sandoz and has received funding for travel and honoraria from Alexion, Merck, Novartis, Sanofi, Roche, and Janssen; J. Ciron has received consulting fees for attending scientific advisory board, lectures or other activities from Biogen, Novartis, Merck, Sanofi, Roche, Alexion, and Horizon Therapeutics—Amgen; P. Clavelou has received consulting and lecturing fees, travel grants, and unconditional research support from Biogen, Janssen, Medday, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva Pharma; J. De Sèze has received consulting and lecturing fees, travel grants, and unconditional research support from Biogen, Genzyme, Novartis, Roche, Sanofi Aventis, and Teva Pharma; G. Defer has received consulting and lecturing fees for Biogen, Novartis, Genzyme, Merck-Serono, Roche, and Teva and funding for travel from Merck Serono, Biogen, Sanofi-Genzyme, Novartis, and Teva, Institution granted for research supporting from Merck Serono, Biogen, Genzyme, and Novartis; I. Doghri has received consulting and non-financial support from Merck, Novartis, Sanofi, and Sandoz; A. Dos Santos has received consulting and lecturing fees, travel grants, or unconditional research support from the following pharmaceutical companies: Alexion, Biogen, Janssen, Merck, Novartis, BMS, Teva, Argenx, and Sanofi; K. Hankiewicz has nothing to disclose; P. Labauge has received consulting and lecturing fees, travel grants, and unconditional research support from Biogen, Genzyme, Novartis, Merck Serono, Roche, and Teva Pharma; E. Le Page has received consulting or lectures, and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis Alexion, research support from Teva and Biogen, academic research grants from PHRC and LFSEP, and travel grant from ARSEP Foundation; L. Magy reports no disclosures relevant to the manuscript; E. Maillard has received consulting and lecturing fees from Alexion, Biogen, Horizon, Janssen, Merck Serono, Novartis, Roche, Sandoz, Sanofi-Genzyme, and Teva Pharmaceuticals, and research support from Biogen; L. Michel has received personal compensation for consulting, speaking, or other activities with Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Teva, and BMS; T. Moreau has received fees as a scientific adviser from Biogen, Medday, Novartis, Genzyme, and Sanofi; S. Moulin has nothing to disclose; J. Pelletier and C. Pottier report no disclosures relevant to the manuscript; A. Ruet has received honoraria for meeting speaking from Merck, Alexion, Horizon Th, and Sanofi Genzyme and has received support for traveling from Biogen, Novartis, and Merck, her institution received research grants from Biogen, Roche, Sanofi-Genzyme, and BMS; M. Sarov-Riviere reports no disclosures relevant to the manuscript; B. Stankoff has received lecturing fees from Biogen Idec, Merck-Serono, Novartis, Sanof, and Janssen, and unconditional research support from Merck-Serono, Novartis, and Roche; E. Thouvenot has received consulting and lecturing fees, travel grants, or unconditional research support from the following pharmaceutical companies: Actelion, Biogen, Janssen, Merck Serono, Novartis, Roche, and Sanofi; A. Wahab has received consulting and lecturing fees from Novartis, Merck, and Roche and travel grants from Novartis and Merck; H. Zephir has received consulting or lectures, and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis, and Bayer, as well as research support from Teva and Roche, and academic research grants from Académie de Médecine, LFSEP, FHU Imminent, and ARSEP Foundation; E. Leray received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Alexion, Biogen, Merck, Novartis, Roche, and Sanofi-Genzyme; D. Laplaud board membership, consultancy, and grants from Alexion, Actelion, BMS, Biogen, Egle Therapeutics, Janssen, Merck, MSD, Novartis, Roche, and Sanofi. Go to
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical