Modulator of VRAC Current 1 Is a Potential Target Antigen in Multiple Sclerosis
- PMID: 39933126
- PMCID: PMC11839221
- DOI: 10.1212/NXI.0000000000200374
Modulator of VRAC Current 1 Is a Potential Target Antigen in Multiple Sclerosis
Abstract
Background and objectives: Multiple sclerosis (MS) is a chronic immune-mediated demyelinating disease of the CNS. Highlighted by the success of B-cell-depleting therapies such as the monoclonal anti-CD20 antibodies rituximab, ocrelizumab, and ofatumumab, B cells have been shown to play a central role in the immunopathology of the disease. Yet, the target antigens of the pathogenic B-cell response in MS remain unclear.
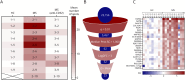
Methods: We combined polyclonal B-cell stimulation of peripheral blood mononuclear cells with a human proteome-wide protein microarray to identify target antigens of MS by comparing samples from 20 patients with MS with 9 age-matched and sex-matched healthy controls. Results were verified by enzyme-linked immunosorbent assay (ELISA) in 3 independent validation cohorts (N = 47 patients with MS in remission; N = 20 patients with MS during relapse; N = 25 HCs; N = 30 patients with other noninflammatory neurologic diseases; N = 9 patients with other inflammatory neurologic diseases). Experimental autoimmune encephalomyelitis (EAE) was used as an animal model to evaluate the pathogenicity of the antibodies of choice.
Results: Our results corroborate the existing concept of a highly diverse autoimmune response in MS. Yet, a significantly elevated antibody response against the membrane protein modulator of VRAC current 1 (MLC1) was noted in B-cell culture supernatants and serum samples of patients with MS. Furthermore, significantly elevated titers to MLC1 were observed in the CSF of patients with neuroinflammatory diseases other than MS. Neurons and astrocytes were identified as the main cell types expressing MLC1 in the brain of a patient with MS. Injection of anti-MLC1 antibodies into mice with EAE led to strong in vivo binding to cerebral cortical neurons and to the death of 4 of the 7 injected mice.
Discussion: Future studies will have to address the diagnostic and prognostic value of MLC1-specific antibodies in neuroinflammatory disorders such as MS and characterize the functional role of MLC1 expression in neurons and astrocytes.
Trial registration information: The study has been registered in the German Clinical Trials Register (study number DRKS00015528).
Conflict of interest statement
A. Weier, J.R. Dahl, M. Hintze, C. Winter, J. Oechtering, J. Kuhle, G. Luber, and T. Heider have declared that no conflict of interest exists. V. Rothhammer was funded by an ERC Starting Grant from the European Research Council (HICI 851693), a Heisenberg Fellowship, and a research grant provided by the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG] RO4866-3/1, RO4866-4/1; project ID 401772351). He was also supported by transregional and collaborative research centers provided by the DFG (project ID 408885537–TRR 274; project ID 261193037–CRC 1181; project ID 505539112–KFO 5024). T. Tsaktanis was funded by the Kommission für Klinische Forschung, Klinikum rechts der Isar, as well as by the Clinician Scientist Program of the Medical Faculty of Friedrich-Alexander-Universität Erlangen-Nürnberg. A-K. Proebstel has received financial compensation for participation in advisory boards and/or consultations from Biogen, Novartis, Roche, and UCB, and research support from Biogen. She has received funding from the Swiss National Science Foundation (Eccellenza Professorship 194609; Starting Grant 211318), the National Multiple Sclerosis Society Kathleen C. Moore Fellowship: FG-1708-28871, the European Union Horizon Europe research and innovation program and Swiss State Secretariat for Education, Research and Innovation (SERI) (101136582), the Propatient Foundation, the Fondation Pierre Mercier pour la science, and the Gottfried & Julia Bangerter-Rhyner-Foundation. T. Neziraj is funded by a Department of Defense Multiple Sclerosis Research Program Early Investigator Research Award (MS220186). B-A. Kallmann has received speaker fees and consultancy honoraria from Hexal, Alexion, Biogen, BMS, Viatris, Novartis, Merck, Janssen, Roche, and Sanofi. L. Klotz has received compensation for serving on scientific advisory boards for Alexion, Genzyme, Janssen, Merck, Novartis, and Roche; speaker honoraria and travel support from Bayer, Biogen, Genzyme, Grifols, Merck, Novartis, Roche, Santhera, and Teva; and research support from the DFG, the German Ministry for Education and Research (BMBF), the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Münster, the research program Innovative Medizinische Forschung (IMF) Münster, Biogen, Novartis, and Merck. R. Chunder has received funding from the DFG under Germany's Excellence Strategy (EXC2151–390873048) and from Novartis (Oppenheim-Förderpreis für Multiple Sklerose 2023). S. Kuerten reports funding from Novartis, F. Hoffmann-La Roche, and Sanofi, and speaker fees and consultancy honoraria from Novartis, F. Hoffmann-La Roche, Sanofi, and Teva. S. Kuerten receives funding from the German Research Foundation (DFG) IRTG 2168 (grant no. 272482170) and from DFG project 460333672 CRC1540 EBM. She is a member of the Excellence Cluster “ImmunoSensation2” (EXC2151–390873048). Go to
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
