Effect of Nitazoxanide and Probiotic Treatment on Bangladeshi Children with Cryptosporidiosis
- PMID: 39933176
- PMCID: PMC11965742
- DOI: 10.4269/ajtmh.23-0914
Effect of Nitazoxanide and Probiotic Treatment on Bangladeshi Children with Cryptosporidiosis
Abstract
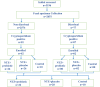
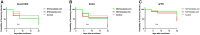
Cryptosporidium spp. is a cause of diarrhea morbidity and mortality in children under 5 years of age. In addition, asymptomatic infections can have a negative impact on growth and development. In low- and middle-income countries where a greater number of infants may be malnourished, the results of treating cryptosporidiosis with the only Food and Drug Administration-approved drug nitazoxanide (NTZ) have been inconsistent. Malnutrition is both a risk factor for cryptosporidiosis and a consequence of infection with this parasite. Treatment with the probiotic Lactobacillus reuteri DSM 17938 has been shown to assist in nutritional recovery and the restoration of gut health. In this pilot randomized clinical trial, we examined whether combined probiotic and NTZ treatment could result in the reduction in parasitemia and infection-associated growth stunting in undernourished children. Cryptosporidium spp.-positive Bangladeshi children with a weight-for-length Z score between -1 and -3 were randomly assigned to one of three groups. Group 1 (n = 26) received NTZ and Lactobacillus, group 2 (n = 28) received NTZ along with a placebo, and the third control group (n = 10) received standard care. There was no difference in the duration of infection or improvement in child anthropometric measurements in any treatment group compared with control. Therefore, this pilot study does not provide support for treatment with NTZ, Lactobacillus, or the two in combination as an effective means of reducing the duration of Cryptosporidium spp. infection or improving growth in growth-stunted children.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

