Impact of physical activity on physical function, mitochondrial energetics, ROS production, and Ca2+ handling across the adult lifespan in men
- PMID: 39933528
- PMCID: PMC11866497
- DOI: 10.1016/j.xcrm.2025.101968
Impact of physical activity on physical function, mitochondrial energetics, ROS production, and Ca2+ handling across the adult lifespan in men
Abstract
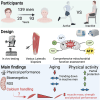
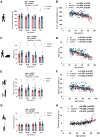
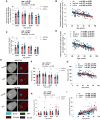
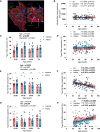
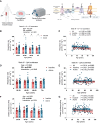
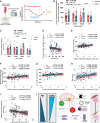
Aging-related muscle atrophy and weakness contribute to loss of mobility, falls, and disability. Mitochondrial dysfunction is widely considered a key contributing mechanism to muscle aging. However, mounting evidence positions physical activity as a confounding factor, making unclear whether muscle mitochondria accumulate bona fide defects with aging. To disentangle aging from physical activity-related mitochondrial adaptations, we functionally profiled skeletal muscle mitochondria in 51 inactive and 88 active men aged 20-93. Physical activity status confers partial protection against age-related decline in physical performance. Mitochondrial respiration remains unaltered in active participants, indicating that aging per se does not alter mitochondrial respiratory capacity. Mitochondrial reactive oxygen species (ROS) production is unaffected by aging and higher in active participants. In contrast, mitochondrial calcium retention capacity decreases with aging regardless of physical activity and correlates with muscle mass, performance, and the stress-responsive metabokine/mitokine growth differentiation factor 15 (GDF15). Targeting mitochondrial calcium handling may hold promise for treating aging-related muscle impairments.
Keywords: calcium retention capacity; functional capacities; intermuscular fat accumulation; mitochondria; mitochondrial permeability transition pore; muscle atrophy and weakness; physical performance; reactive oxygen species; sarcopenia; skeletal muscle aging.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. - DOI - PMC - PubMed
-
- Janssen I., Heymsfield S.B., Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002;50:889–896. - PubMed
-
- Janssen I., Baumgartner R.N., Ross R., Rosenberg I.H., Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004;159:413–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

