Repeated evolution of photoperiodic plasticity by different genetic architectures during recurrent colonizations in a butterfly
- PMID: 39933585
- PMCID: PMC11813577
- DOI: 10.1098/rspb.2024.2195
Repeated evolution of photoperiodic plasticity by different genetic architectures during recurrent colonizations in a butterfly
Abstract
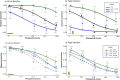
In cases of recurrent colonizations of similar habitats from the same base population, it is commonly expected that repeated phenotypic adaptation is caused by parallel changes in genetic variation. However, it is becoming increasingly clear that similar phenotypic variation may also evolve by alternative genetic pathways. Here, we explore the repeated evolution of photoperiodic plasticity for diapause induction across Swedish populations of the speckled wood butterfly, Pararge aegeria. This species has colonized Scandinavia at least twice, and population genomic results show that one of the candidate regions associated with spatial variation in photoperiodism is situated on the Z-chromosome. Here, we assay hybrid crosses between several populations that differ in photoperiodic plasticity for sex-linked inheritance of the photoperiodic reaction norm. We find that while a cross between more distantly related populations from the two different colonization events shows strong sex-dependent inheritance of photoperiodic plasticity, a cross between two more closely related populations within the oldest colonization range shows no such effect. We conclude that the genotype-phenotype map for photoperiodic plasticity varies across these populations and that similar local phenotypic adaptation has evolved during recurrent colonization events by partly non-parallel genetic changes.
Keywords: Z-linkage; colonization; diapause; genotype-phenotype map; parallel evolution; plasticity.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Haldane JBS. 1932. The causes of evolution. London, UK: Longman.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

