Phase 1 study of novel anti-platelet agent to overcome pharmacogenomic limitations of clopidogrel
- PMID: 39933830
- PMCID: PMC11815446
- DOI: 10.1136/openhrt-2024-003088
Phase 1 study of novel anti-platelet agent to overcome pharmacogenomic limitations of clopidogrel
Abstract
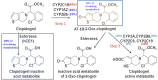
Aims: Clopidogrel is the most commonly prescribed thienopyridine as part of dual anti-platelet therapy for the treatment of cardiovascular diseases. However, clopidogrel responsiveness shows variability based on CYP2C19 polymorphism. Therefore, we planned a study with an objective of evaluating safety, tolerability, pharmacodynamics and pharmacokinetics of a novel thienopyridine antiplatelet agent AT-10 in healthy Indian subjects compared with standard dosage regimen of clopidogrel based on their CYP2C19 genotyping.
Methods: Two CYP2C19 genotype-based groups were identified, that is, poor metabolisers and extensive metabolisers, with 20 subjects in each group (n=40) for participating in a randomised, two-period, crossover study. Each study period lasted 6 days including administration of loading and maintenance doses of AT-10 (40 mg/10 mg) or clopidogrel (300 mg/75 mg). The pharmacokinetics and pharmacodynamics were assessed on day 1 and day 6 at several time intervals.
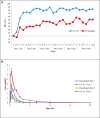
Results: Overall result of pharmacodynamic parameters showed that mean %inhibition of platelet aggregation between AT-10 and clopidogrel in all subjects at 6 hours postdose (loading dose) (AT-10: clopidogrel; 73.30% vs 18.53%) and 6 hours postdose on day 6 (maintenance dose) (AT-10: clopidogrel; 83.41% vs 51.19 %) obtained from the AT-10 group was significantly higher than the clopidogrel group. Further, %inhibition of platelet aggregation from AT-10 treatment in poor metaboliser group was significantly higher than the clopidogrel treatments in extensive metaboliser group.Overall pharmacokinetic comparison in all subjects indicates that AT-10 gives greater exposure to active Metabolite H4 than clopidogrel.
Conclusion: AT-10 showed better inhibition of platelet aggregation in poor metabolizers as compared to Clopidogrel. AT-10 may emerge as a potential alternative to Clopidogrel as an anti-platelet drug. It can be further developed in clinical studies for the unmet medical needs in management of CVDs and overcome the pharmacogenomic limitations of Clopidogrel.
Trial registration number: Clinical Trial Registry-India URL: http://ctri.nic.in.
Registration number: CTRI/2021/03/032206.
Keywords: Acute Coronary Syndrome; Genetics; Pharmacology, Clinical; Translational Medical Research.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AP is the President of Medical Affairs and Clinical Research Department in the pharmaceutical company Ipca Laboratories Limited. NC is the Senior Vice President of Clinical Research and Development in the pharmaceutical company Ipca Laboratories Limited. VR is currently the Deputy General Manager in Clinical Research and Development department at Ipca Laboratories Limited. KN is the Assistant General Manager in Clinical Data Management and Statistical Analysis at Ipca Laboratories Limited. All authors are employees of Ipca Laboratories Limited that developed AT-10, and all authors were involved in conceptualisation, coordination and execution of the study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical