4-1BB agonist targeted to fibroblast activation protein α synergizes with radiotherapy to treat murine breast tumor models
- PMID: 39933836
- PMCID: PMC11815443
- DOI: 10.1136/jitc-2024-009852
4-1BB agonist targeted to fibroblast activation protein α synergizes with radiotherapy to treat murine breast tumor models
Abstract
Background: Ionizing radiation (IR) is a double-edged sword for immunotherapy as it may have both immunosuppressive and immunostimulatory effects. The biological effects of IR on the tumor microenvironment (TME) are a key factor for this balance. Fibroblast activation protein (FAP) is expressed on the surface of cancer-associated fibroblasts (CAF) in many cancer types and its abundance is associated with the poor immune response to immune-checkpoint-blockade in patients. We hypothesized that IR increases FAP expression in CAFs, therefore the combination of IR with targeted immunomodulators such as an agonistic anti-FAP-4-1BBL fusion protein could enhance the immune-mediated antitumoral effects of these treatments.
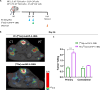
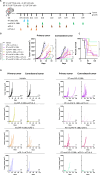
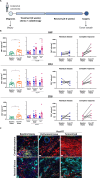
Methods: The murine transplantable TS/A tumor-cell-line co-engrafted with CAFs was used to investigate increases in FAP expression in tumors following irradiation using immunohistochemistry, real-time polymerase chain reaction (RT-PCR) and multiplex tissue immunofluorescence. One lesion of bilateral tumor-bearing mice was only locally irradiated or combined with weekly injections of the bispecific muFAP-4-1BBL fusion protein (a mouse surrogate for RG7826). Tumor sizes were followed over time and TME was assessed by flow cytometry. Selective monoclonal antibody (mAb)-mediated depletions of immune cell populations, neutralizing interferon alpha/beta receptor 1 (IFNAR-I) IFNAR and interferon (IFN)-γ mAbs and gene-modified mice (4-1BB-/-) were used to delineate the immune cell subsets and mechanisms required for efficacy. 67Ga labeled muFAP-4-1BBL tracked by SPECT-CT was used to study biodistribution. In human colorectal carcinoma samples, the inducibility of FAP expression following radiotherapy was explored by multiplex immunofluorescence.
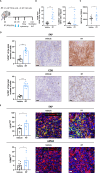
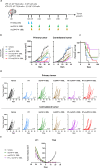
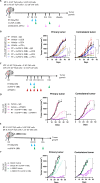
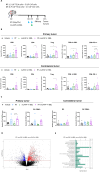
Results: Irradiation of TS/A+CAF tumors in mice showed an increase in FAP levels after local irradiation. A suboptimal radiotherapy regimen in combination with muFAP-4-1BBL attained primary tumor control and measurable abscopal effects. Immune TME landscape analyses showed post-treatment increased infiltration of activated immune cells associated with the combined radioimmunotherapy treatment. Efficacy depended on CD8+ T cells, type I IFN, IFN-γ and ability to express 4-1BB. Biodistribution studies of muFAP-4-1BBL indicated enriched tumor targeting to irradiated tumors. Human colorectal cancer samples pre and post irradiation showed enhanced FAP expression after radiotherapy.
Conclusion: Increased FAP expression in the TME as a result of radiotherapy can be exploited to target agonist 4-1BB immunotherapy to malignant tumor lesions using an FAP-4-1BBL antibody fusion protein.
Keywords: Abscopal; Monoclonal antibody; Radiotherapy/radioimmunotherapy; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: EG-S, IS-M, LF-R, AR, CEDA, CB-A, CdPH, CLR, AT, JAS, MC, and PS-M declare no competing interests. MER-R reports receiving research funding from Roche and Highlight Therapeutics. She also has received speaker’s bureau honoraria from BMS and ROCHE. IM reports receiving commercial research grants from BMS, Highlight Therapeutics, Alligator, Pfizer Genmab and Roche; has received speaker’s bureau honoraria from MSD; and is consultant or advisory board member for BMS, Roche, AstraZeneca, Genmab, Pharmamar, F-Star, Bioncotech, Bayer, Numab, Pieris, Gossamer, Alligator and Merck Serono. CK, PU, CC, and TT declare employment, stock ownership and patents with Roche.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
