Spatiotemporal Study of a Risk-Stratification Epigenetic-Based Biomarker Assay in Patients With Barrett Esophagus
- PMID: 39933887
- PMCID: PMC12124209
- DOI: 10.14309/ajg.0000000000003367
Spatiotemporal Study of a Risk-Stratification Epigenetic-Based Biomarker Assay in Patients With Barrett Esophagus
Abstract
Introduction: Barrett esophagus (BE) is the strongest known risk factor for developing esophageal adenocarcinoma (EAC), the second-most lethal cancer in the United States. Esopredict is a novel validated methylation-based biomarker assay that provides precise quantification of neoplastic progression risk in BE patients. Inherit challenges, including tissue heterogeneity, sampling error, interobserver variability, and inconsistent adherence to surveillance biopsy guidelines, may affect the predictive value results of Esopredict obtained at different anatomic locations or different sampling time points.
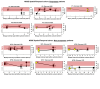
Methods: To investigate the spatiotemporal performance of Esopredict across multiple spatiotemporal sampling points, we profiled 220 biopsies obtained from 58 BE patients, including 11 patients with overlapping spatial and temporal biopsies. We focused on spatial profiling (i.e., multiple biopsies obtained at several anatomic locations during a single endoscopy) and temporal profiling (i.e., biopsies obtained from multiple endoscopies performed at different time points). Each patient had an initial histologic diagnosis of nondysplastic Barrett esophagus, indefinite for dysplasia, or low-grade dysplasia. Final follow-up (endpoint) biopsies showed either high-grade dysplasia or EAC (progressors), or nondysplastic Barrett esophagus, indefinite for dysplasia, or low-grade dysplasia (nonprogressors). Biopsies were analyzed with Esopredict to compute a progression risk score, which quantified the likelihood of future progression to high-grade dysplasia or EAC within 5 years.
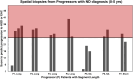
Results: In 52 spatially profiled patients, Esopredict demonstrated a sensitivity of 81% (17/21 progressor patients), based on the highest-scoring biopsy from each patient; sensitivity increased to 100% (12/12) when end point biopsies occurred within 5 years of the index (initial) biopsy. In 28 temporally profiled patients, sensitivity was 100% (8/8 patients), based on the biopsy performed at the time point closest to the end point biopsy.
Discussion: Esopredict showed high predictive performance in multiple spatiotemporal samples in BE patients. These data further support the use of Esopredict as a robust test to distinguish high-risk BE patients, who may benefit from endoscopic eradication therapy or increased surveillance frequency, from low-risk patients, who may be candidates for less frequent surveillance and noninterventional observation.
Keywords: biopsy assay; clinical decisions; epigenetics; esophageal adenocarcinoma; personomics; predictive biomarkers; prognostic assay.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures




References
-
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365(15):1375–83. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clinic 2021;71(3):209–49. - PubMed
-
- Surveillance, Epidemiology, and End Results (SEER) Program . SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969-2019) <Katrina/Rita Population Adjustment. National Cancer Institute, DCCPS, Surveillance Research Program; www.seer.cancer.gov (2021, Accessed 2022).
-
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol 2021;18(6):432–43. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

