The Electrically Silent Kv5.1 Subunit Forms Heteromeric Kv2 Channels in Cortical Neurons and Confers Distinct Functional Properties
- PMID: 39933932
- PMCID: PMC11949482
- DOI: 10.1523/JNEUROSCI.2293-23.2025
The Electrically Silent Kv5.1 Subunit Forms Heteromeric Kv2 Channels in Cortical Neurons and Confers Distinct Functional Properties
Abstract
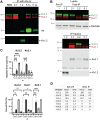
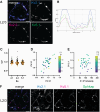
Voltage-gated K+ channels of the Kv2 family are highly expressed in brain and play dual roles in regulating neuronal excitability and in organizing endoplasmic reticulum-plasma membrane (ER-PM) junctions. Studies in heterologous cells suggest that Kv2.1 and Kv2.2 co-assemble with "electrically silent" KvS subunits to form heterotetrameric channels with distinct biophysical properties, but the prevalence and localization of these channels in native neurons are unknown. Here, using mass spectrometry-based proteomics, we identified five KvS subunits as components of native Kv2.1 channels immunopurified from mouse brain of both sexes, the most abundant being Kv5.1. We found that Kv5.1 co-immunoprecipitates with Kv2.1 and to a lesser extent with Kv2.2 from brain lysates and that Kv5.1 protein levels are decreased by 70% in Kv2.1 knock-out mice and 95% in Kv2.1/Kv2.2 double knock-out mice. RNAscope and immunolabeling revealed that Kv5.1 is prominently expressed in neocortex, where it is detected in a substantial fraction of Kv2.1/Kv2.2 positive neurons in layers 2/3, 5, and 6. At the subcellular level, Kv5.1 protein is coclustered with Kv2.1 and Kv2.2 at presumptive ER-PM junctions on the soma and proximal dendrites of cortical neurons. Moreover, in addition to modifying channel conductance, we found that Kv2/Kv5.1 channels are less phosphorylated and insensitive to RY785, a potent and selective Kv2 channel inhibitor. Together, these findings demonstrate that KvS subunits create multiple Kv2 channel subtypes in brain. Most notably, Kv2/Kv5.1 channels are highly expressed in cortical neurons, where their unique properties likely modulate the critical conducting and nonconducting roles of Kv2 channels.
Keywords: ER-PM junction; channel localization; modulatory subunit; voltage-gated potassium channel.
Copyright © 2025 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures









Update of
-
Electrically silent KvS subunits associate with native Kv2 channels in brain and impact diverse properties of channel function.bioRxiv [Preprint]. 2024 Jan 25:2024.01.25.577135. doi: 10.1101/2024.01.25.577135. bioRxiv. 2024. Update in: J Neurosci. 2025 Mar 26;45(13):e2293232025. doi: 10.1523/JNEUROSCI.2293-23.2025. PMID: 38328147 Free PMC article. Updated. Preprint.
References
-
- Bishop HI, Guan D, Bocksteins E, Parajuli LK, Murray KD, Cobb MM, Misonou H, Zito K, Foehring RC, Trimmer JS (2015) Distinct cell- and layer-specific expression patterns and independent regulation of Kv2 channel subtypes in cortical pyramidal neurons. J Neurosci 35:14922–14942. 10.1523/JNEUROSCI.1897-15.2015 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
