Lactate and lactylation in cancer
- PMID: 39934144
- PMCID: PMC11814237
- DOI: 10.1038/s41392-024-02082-x
Lactate and lactylation in cancer
Abstract
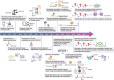
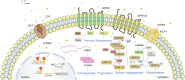
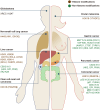
Accumulated evidence has implicated the diverse and substantial influence of lactate on cellular differentiation and fate regulation in physiological and pathological settings, particularly in intricate conditions such as cancer. Specifically, lactate has been demonstrated to be pivotal in molding the tumor microenvironment (TME) through its effects on different cell populations. Within tumor cells, lactate impacts cell signaling pathways, augments the lactate shuttle process, boosts resistance to oxidative stress, and contributes to lactylation. In various cellular populations, the interplay between lactate and immune cells governs processes such as cell differentiation, immune response, immune surveillance, and treatment effectiveness. Furthermore, communication between lactate and stromal/endothelial cells supports basal membrane (BM) remodeling, epithelial-mesenchymal transitions (EMT), metabolic reprogramming, angiogenesis, and drug resistance. Focusing on lactate production and transport, specifically through lactate dehydrogenase (LDH) and monocarboxylate transporters (MCT), has shown promise in the treatment of cancer. Inhibitors targeting LDH and MCT act as both tumor suppressors and enhancers of immunotherapy, leading to a synergistic therapeutic effect when combined with immunotherapy. The review underscores the importance of lactate in tumor progression and provides valuable perspectives on potential therapeutic approaches that target the vulnerability of lactate metabolism, highlighting the Heel of Achilles for cancer treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Not applicable. Declaration of animal ethics approval: Studies cited in this review involving experiments with animals are all equipped with animal care in accordance with institution guidelines.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

