Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial
- PMID: 39934669
- PMCID: PMC11818026
- DOI: 10.1186/s12879-025-10584-4
Prophylaxis for renal patients at risk of COVID-19 infection: results from the intranasal niclosamide randomised, double blinded, placebo controlled arm of the PROTECT-V platform trial
Abstract
Purpose: Despite vaccination, many patients remain vulnerable to COVID-19 infection and poorer outcomes, because of underlying health conditions resulting in sub-optimal vaccine responses. This study aims to demonstrate whether intranasal niclosamide confers additional protection against COVID-19 infection above standard preventative measures including vaccination.
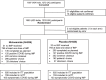
Methods: PROTECT-V (PROphylaxis for paTiEnts at risk of COVID-19 infecTion) is a platform trial testing multiple pre-exposure COVID-19 prophylactic agents in vulnerable patients. This paper reports results from the randomised, double blind, placebo controlled intranasal niclosamide arm. 1651 adult patients on dialysis, with a kidney transplant or renal autoimmune conditions on immunosuppression were randomised from 48 sites (37 UK; 11 Indian). Intranasal niclosamide or matched placebo was administered twice daily, for up to nine months. Primary outcome was time to symptomatic COVID-19 infection.
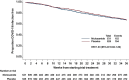
Results: 1651 patients were randomised (826 niclosamide;825 placebo) between February 2021 to November 2022. 655(39.7%) were dialysis patients, 622(37.7%) kidney transplant recipients and 374(22.7%) had renal autoimmune disease. 97.5% patients in the UK and 66.4% patients in India with comparable proportions in both treatment groups had received COVID-19 vaccinations. Despite no adverse safety signal, there was a high withdrawal rate (40% niclosamide;23.8% placebo) due to local upper airway irritation leading to a significantly shorter treatment duration in the niclosamide group). Symptomatic COVID-19 infection during study treatment was observed in 103 patients in the niclosamide group and 133 in the placebo group (estimated hazard ratio 1.02(95%CI 0.79-1.32)).
Conclusion: Intranasal niclosamide did not reduce risk of symptomatic COVID-19 infection in this cohort compared to placebo.
Trial registration: This study is registered with ClinicalTrials.gov: NCT04870333 (submitted 01/03/2021; posted 03/05/2021), EudraCT: 2020-004144-28 and the Clinical Trials Registry of India (CTRI):#CTRI/2022/03/040802.
Keywords: COVID-19; Clinical trial; Prophylaxis; Renal.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approvals and consent to participate: The protocol was approved by the UK Medicines and Healthcare Products Regulatory Agency, South Central Berkshire Research Ethics Committee (REC Reference 20/SC/0403) in the UK and the Central Drugs Standard Control Organisation, India and the Ethics Committees of all participating sites in India. Consent for publication: Not applicable. Competing interest: All authors have completed the Unified Competing Interest Form (available on request from the corresponding author) and declare the following: MCX has received PhD funding support from GlaxoSmithKline. ID has research grants from Sanofi, Baxter and NIHR CRN West Midlands; honoraria from GSK, Sanofi, Vifor and AstraZeneca; support to attend meetings from GlaxoSmithKline; and sits on DSMB/advisory boards for GlaxoSmithKline and Vifor. DD has received research grants from GlaxoSmithKline; sites on DSMB/advisory boards for Gilead Sciences Inc and Synairgen Plc and since May 2023 is an employee of AstraZeneca. MJ, PS and AWC are employees of Union therapeutics A/S. MLJ is an employee of Union therapeutics A/S and sits on the advisory board for Radiometer and board of directors for HEDIA. MAOS is a co-founder and shareholder at Union therapeutics A/S and a co-inventor on patents and patent applications around the use of niclosamide to treat COVID-19. VJ has received consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Bayer, Vera, Visterra, Biocryst, AstraZeneca; honoraria from Baxter Healthcare and GlaxoSmithKline; and sits on DSMB/advisory boards for Zydus Lifesciences. TFH is and employee and shareholder at GlaxoSmithKline and is an ICH E20 working Group Member. RMS has received research grants from LifeArc, Kidney Research UK, Addenbrooke’s Charitable Trust, Union therapeutics A/S and GlaxoSmithKline for the PROTECT-V trial. TJLH, WQ, FD, RA, TH, LS, AB, NB, JRB have no conflicts of interest to declare.
Figures


References
-
- Weiss A, Hendrickx R, Stensgaard E, Jellingsø M, Sommer MOA. Kidney Transplant and Dialysis Patients Remain at Increased Risk for Succumbing to COVID-19. Transplantation. 2023;107(5):1136–8. Available from: https://journals.lww.com/10.1097/TP.0000000000004462 - DOI - PMC - PubMed
-
- Krueger KM, Ison MG, Ghossein C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am J Kidney Dis. 2020;75(3):417–25. Available from: http://www.ajkd.org/article/S0272638619308911/fulltext. [cited 2022 Oct 8]. - PubMed
-
- Bingham CO, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. - PubMed
-
- Van Assen S, Holvast A, Benne CA, Posthumus MD, Van Leeuwen MA, Voskuyl AE, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/art.25033 . [cited 2023 Mar 25]. - DOI - PubMed
-
- Caillard S, Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17(12):785–7. Available from: https://pubmed.ncbi.nlm.nih.gov/34580488/. [cited 2022 Oct 8]. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

