The impact of long-acting Gonadotropin-releasing hormone agonist pretreatment on the clinical pregnancy outcomes of hormone replacement therapy-frozen embryo transfer in recurrent implantation failure patients with and without polycystic ovary syndrome: a retrospective clinical study
- PMID: 39934706
- PMCID: PMC11817544
- DOI: 10.1186/s12884-025-07264-1
The impact of long-acting Gonadotropin-releasing hormone agonist pretreatment on the clinical pregnancy outcomes of hormone replacement therapy-frozen embryo transfer in recurrent implantation failure patients with and without polycystic ovary syndrome: a retrospective clinical study
Abstract
Background: Several studies have demonstrated that pre-treatment with long-acting Gonadotropin-Releasing Hormone agonists (GnRHa) can significantly enhance the clinical pregnancy rate among recurrent implantation failure (RIF) patients. Investigations have also suggested that GnRHa pre-treatment could ameliorate the clinical pregnancy and live birth rates in polycystic ovary syndrome (PCOS) patients. But there is a dearth of research on whether long-acting GnRHa pre-treatment yields superior clinical outcomes for RIF patients with PCOS.
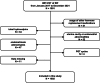
Methods: The retrospective study enrolled 1602 patients under the age of 40 meeting the criteria for RIF at the Reproductive Medicine Center of Nanjing Drum Tower Hospital, who underwent frozen-thawed embryo transfer (FET) between January 2017 and December 2021. All cycles were categorized into hormone replacement therapy (HRT) Group (n = 1283) and GnRHa-HRT Group (n = 319), contingent on the usage of long-acting GnRHa pretreatment. Primary outcomes investigated in this study was clinical pregnancy rate, while live birth rate and early miscarriage rate were deemed as secondary outcomes. Univariate analysis and a multivariate logistic regression model were employed to assess the impact of GnRHa pretreatment on the clinical pregnancy rate in RIF patients. The influence of long-acting GnRHa pretreatment on clinical pregnancy outcomes was re-examined in PCOS and non-PCOS subgroups. Additionally, an interaction analysis was performed to evaluate the effect of PCOS on the relationship between long-acting GnRHa pretreatment and the clinical pregnancy rate.
Results: Multiple regression analysis showed that long-acting GnRHa pretreatment had a positive impact on the clinical pregnancy rate (aOR = 1.51, 95%CI: 1.15-1.99, P = 0.003). We divided the RIF population into two subgroups, for PCOS patients, although the clinical pregnancy rate was higher in women who received GnRHa pretreatment compared to those who did not, it was not statistically significant (aOR = 1.51, 95%CI: 0.81-2.82, P = 0.195). Interaction analysis suggested that for PCOS patients, there was no significant difference in the clinical pregnancy rate between women who received GnRHa pretreatment and those who did not (P interaction = 0.818), indicating that the effect of GnRHa pretreatment on the clinical pregnancy rate was not influenced by PCOS.
Conclusions: Our study demonstrates that long-acting GnRHa pretreatment can enhance clinical pregnancy outcomes in patients with RIF. Among RIF patients without PCOS, the clinical pregnancy rate exhibited a significant increase following GnRHa pretreatment compared to the control group. However, in RIF patients with concurrent PCOS, there was no significant elevation in the clinical pregnancy rate post-GnRHa pretreatment. Therefore, GnRHa pretreatment is effective in improving pregnancy outcomes for RIF patients. However, whether GnRHa pretreatment is suitable for RIF patients with PCOS requires more cautious clinical discussion.
Keywords: Clinical pregnancy rate; Interaction analysis; Long-acting GnRHa; PCOS; RIF.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This retrospective study received ethical approval from the ethics committee of Nanjing Drum Tower Hospital. All methods were carried out in accordance with relevant guidelines and regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Pretreatment with a GnRH agonist and hormone replacement treatment protocol could not improve live birth rate for PCOS women undergoing frozen-thawed embryo transfer cycles.BMC Pregnancy Childbirth. 2021 Dec 18;21(1):835. doi: 10.1186/s12884-021-04293-4. BMC Pregnancy Childbirth. 2021. PMID: 34922467 Free PMC article.
-
Pretreatment with a long-acting GnRH agonist for frozen-thawed embryo transfer cycles: how to improve live birth?J Ovarian Res. 2023 Sep 25;16(1):197. doi: 10.1186/s13048-023-01277-0. J Ovarian Res. 2023. PMID: 37743479 Free PMC article.
-
GnRH-a use before programmed frozen embryo transfer cycles for women with PCOS: a retrospective cohort study.Reprod Biol Endocrinol. 2025 May 15;23(1):72. doi: 10.1186/s12958-025-01403-1. Reprod Biol Endocrinol. 2025. PMID: 40375233 Free PMC article.
-
Effects of gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes in artificial cycles: a meta-analysis.Arch Gynecol Obstet. 2023 Sep;308(3):675-683. doi: 10.1007/s00404-022-06823-7. Epub 2022 Oct 20. Arch Gynecol Obstet. 2023. PMID: 36266549 Review.
-
Effect of Gonadotropin-Releasing Hormone Agonist Pre-Treatment on Outcomes of Fresh and Frozen Embryo Transfers in Women With Adenomyosis: A Retrospective Cohort Study With Literature Review.BJOG. 2025 Apr;132 Suppl 2:62-74. doi: 10.1111/1471-0528.18026. Epub 2024 Dec 17. BJOG. 2025. PMID: 39688600 Review.
Cited by
-
Predictive value of serum sortilin, HMGB1, and galanin-like peptide for gestational diabetes mellitus in women with polycystic ovary syndrome.Front Endocrinol (Lausanne). 2025 May 19;16:1602622. doi: 10.3389/fendo.2025.1602622. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40458174 Free PMC article.
References
-
- Somigliana E, Busnelli A, Kalafat E, Vigano P, Ata B. Recurrent implantation failure: a plea for a widely adopted rational definition. Reprod Biomed Online. 2022;45(2):183–5. - PubMed
-
- Coughlan C, Walters S, Ledger W, Li TC. A comparison of psychological stress among women with and without reproductive failure. Int J Gynaecol Obstet. 2014;124(2):143–7. - PubMed
MeSH terms
Substances
Grants and funding
- BJHPA-2022-SHZHYXZHQNYJ-LCH-002, BJHPA-2023-SZHYXZHQN-006/Fertility Research Program of Young and Middle-aged Physicians-Clinical Research In 2022 and 2023 (Beijing Health Promotion Association)
- 82071646/National Natural Science Foundation of China
- FYX202203/Scientific research project of Jiangsu Province Association of Maternal and Child Health
- JNYYZXKY202213/Open project of the Affiliated Jiangning Hospital of Nanjing Medical University Immune Cell Transformation Research Center
- 2022-YXZX-FC-03/Nanjing Drum Tower Hospital Medical Center Project
LinkOut - more resources
Full Text Sources
Medical