Association of oral anticoagulants with risk of brain haemorrhage expansion compared to no-anticoagulation
- PMID: 39934933
- PMCID: PMC11921975
- DOI: 10.1186/s42466-024-00358-9
Association of oral anticoagulants with risk of brain haemorrhage expansion compared to no-anticoagulation
Abstract
Background: The impact of direct oral anticoagulants (DOAC) on haematoma size after intracerebral haemorrhage (ICH) compared to no-anticoagulation is controversial and prospective data are lacking.
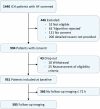
Methods: The investigator-initiated, multicentre, prospective RASUNOA-prime study enrolled patients with non-traumatic ICH and atrial fibrillation while on a DOAC, vitamin K antagonist (VKA) or no anticoagulation (non-OAC). Neuroimaging was reviewed centrally blinded to group allocation. Primary endpoint was haematoma expansion (≥ 6.5 ml or ≥ 33%, any new intraventricular blood or an increase in modified Graeb score by ≥ 2 points) between baseline and follow-up scan within 72 h after symptom onset.
Results: Of 1,440 patients screened, 951 patients with ICH symptom onset less than 24 h before admission were enrolled. Baseline scans were performed at a median of 2 h (IQR 1-6) after symptom onset. Neurological deficit and median baseline haematoma volumes (11 ml; IQR 4-39) did not differ among 577 DOAC, 251 VKA and 123 non-OAC patients. Haematoma expansion was observed in DOAC patients in 142/356 (39.9, 95%-CI 34.8-45.0%), VKA in 47/155 (30.3, 95-CI 23.1%-37.6%), versus non-OAC in 22/74 (29.7, 19.3-40.1%). Unspecific reversal agents in DOAC-ICH (212/356, 59.6%) did not affect the haematoma expansion rate compared to no-antagonization.
Conclusion: Baseline haematoma volume and risk of haematoma expansion did not differ statistically significantly in patients with and without DOAC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The ethics committee of the Medical Faculty of the University Heidelberg as well as all local ethics committees approved the study protocol (S-088/2015). Patients were eligible for the study if they were 18 years or older and either the patient or a legal representative had provided consent. Informed consent could be waived if patients were not able to consent and died before a legal representative could be installed. Consent for publication: Not applicable. Competing interests: RV is an investigator of the NIHR Imperial Biomedical Research Centre and reports fees for consulting and speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Pfizer, Daichi Sankyo, Portola, Biogen, Medtronic, and Morphosys; and research grants from Bayer, Boehringer, Bristol Myers Squibb, Pfizer, Daiichi Sankyo, and Medtronic (ODEA study), outside of the submitted work. TR received consulting honoraria, speakers’ honoraria and travel support from BMS Pfizer, Boehringer Ingelheim, Bayer HealthCare and Daiichi Sankyo, outside the submitted work. WP received consulting honoraria, speakers’ honoraria and travel support from Alexion, Boehringer Ingelheim, Bayer HealthCare and Daiichi Sankyo, outside the submitted work. KA has received lecture fees from Boehringer Ingelheim, Daiichi Sankyo, Alexion and BMS/Pfizer, outside the submitted work. PS received honoraria from Astra Zeneca, Boehringer Ingelheim, Biogen, Daiichi, Amgen, Merck and participated on advisory boards for Boehringer Ingelheim and Astra Zeneca. BG has received speaking honoraria and travel expenses from Bayer Vital GmbH, Boehringer Ingelheim Pharma GmbH & Co.KG, Daiichi Sankyo Deutschland GmbH, UCB Pharma GmbH, UNEEG medical DE GmbH and BMS/Pfizer Alliance, outside the submitted work. GR has received consultation fees and travel expenses from Bayer, Boehringer Ingelheim, Daiichi Sankyo, BMS/Pfizer, Astra Zeneca, Novartis and Cardinal Health, outside the submitted work. DGN has received speaker honoraria, consultation fees and travel expenses from Astra Zeneca, Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Sanofi, outside the submitted work. KGH reports speaker´s honoraria, consulting fees, lecture honoraria and/or study grants from Abbott, Alexion, Amarin, AstraZeneca, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Medronic, Novartis, Pfizer, Portola, Premier Research, Sanofi, SUN Pharma, and W.L. Gore and Associates. CHN received research grants from the German Ministry of Research and Education, German Center for Neurodegenerative Diseases, German Center for Cardiovascular Research, and speaker and/or consultation fees from Abbott, Alexion, Astra Zeneca, Bayer Pharma, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Pfizer Pharma, Portola and Takeda, all outside the submitted work. MEW has received honoraria for lectures from Daiichi Sankyo, BMS/Pfizer and Sanofi. SP has received research support from BMS/Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, European Union, German Federal Joint Committee Innovation Fund, and German Federal Ministry of Education and Research, Helena Laboratories and Werfen as well as speakers’ honoraria/consulting fees from Alexion, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS/Pfizer, Daiichi Sankyo, Portola, and Werfen (all outside the submitted work). PUH reports grants from the German Ministry of Research and Education, European Union, German Parkinson Society, University Hospital Würzburg, the German Heart Foundation, Federal Joint Committee within the Innovationfond, German Research Foundation, Bavarian State, German Cancer Aid, Charité–Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), Bristol-Myers Squibb, Boehringer-Ingelheim, and Daiichi Sankyo), outside the submitted work. JP has received consultation fees and travel expenses from Abbott, Akcea, Bayer, Boehringer Ingelheim, Daiichi Sankyo, and BMS/Pfizer, outside the submitted work. All other authors: none.
References
-
- Patel, M. R., Mahaffey, K. W., Garg, J., Pan, G., Singer, D. E., Hacke, W., Breithardt, G., Halperin, J. L., Hankey, G. J., Piccini, J. P., et al. (2011). Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England Journal of Medicine,365(10), 883–891. - PubMed
-
- Tsivgoulis, G., Wilson, D., Katsanos, A. H., Sargento-Freitas, J., Marques-Matos, C., Azevedo, E., Adachi, T., von der Brelie, C., Aizawa, Y., Abe, H., et al. (2018). Neuroimaging and clinical outcomes of oral anticoagulant-associated intracerebral hemorrhage. Annals of Neurology,84(5), 694–704. - PubMed
-
- Purrucker, J. C., Haas, K., Rizos, T., Khan, S., Wolf, M., Hennerici, M. G., Poli, S., Kleinschnitz, C., Steiner, T., Heuschmann, P. U., et al. (2016). Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurology,73(2), 169–177. - PubMed
-
- Held, C., Hylek, E. M., Alexander, J. H., Hanna, M., Lopes, R. D., Wojdyla, D. M., Thomas, L., Al-Khalidi, H., Alings, M., Xavier, D., et al. (2015). Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: Insights from the ARISTOTLE trial. European Heart Journal,36(20), 1264–1272. - PubMed
-
- Hankey, G. J., Stevens, S. R., Piccini, J. P., Lokhnygina, Y., Mahaffey, K. W., Halperin, J. L., Patel, M. R., Breithardt, G., Singer, D. E., Becker, R. C., et al. (2014). Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: The rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke,45(5), 1304–1312. - PubMed
LinkOut - more resources
Full Text Sources