Intercellular mitochondrial transfer contributes to microenvironmental redirection of cancer cell fate
- PMID: 39934946
- PMCID: PMC12062771
- DOI: 10.1111/febs.70002
Intercellular mitochondrial transfer contributes to microenvironmental redirection of cancer cell fate
Abstract
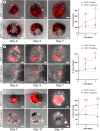
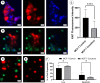
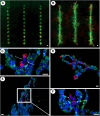
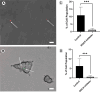
The mammary microenvironment has been shown to suppress tumor progression by redirecting cancer cells to adopt a normal mammary epithelial progenitor fate in vivo. However, the mechanism(s) by which this alteration occurs has yet to be defined. Here, we test the hypothesis that mitochondrial transfer from normal mammary epithelial cells to breast cancer cells plays a role in this redirection process. We evaluate mitochondrial transfer in 2D and 3D organoids using our unique 3D bioprinting system to produce chimeric organoids containing normal and cancer cells. We demonstrate that breast cancer tumoroid growth is hindered following interaction with mammary epithelial cells in both 2D and 3D environments. Furthermore, we show mitochondrial transfer occurs between donor mammary epithelial cells and recipient cancer cells primarily through tunneling nanotubes (TNTs) with minimal amounts seen from extracellular transfer of mitochondria, likely via extracellular vesicles (EVs). This organelle exchange results in various cellular and metabolic alterations within cancer cells, reducing their proliferative potential, and making them susceptible to microenvironmental control. Our results demonstrate that mitochondrial transfer contributes to microenvironmental redirection of cancer cells through alteration of metabolic and molecular functions of the recipient cancer cells. To the best of our knowledge, this is the first description of a 3D bioprinter-assisted organoid system for studying mitochondrial transfer. These studies are also the first mechanistic insights into the process of mammary microenvironmental redirection of cancer and provide a framework for new therapeutic strategies to control cancer.
Keywords: 3D bioprinting; breast cancer; cellular redirection; microenvironment; mitochondrial transfer.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

