Highly Emissive Colloidal Nanocrystals of a "2.5-Dimensional" Monomethylhydrazinium Lead Bromide
- PMID: 39935343
- PMCID: PMC11869272
- DOI: 10.1021/jacs.4c16698
Highly Emissive Colloidal Nanocrystals of a "2.5-Dimensional" Monomethylhydrazinium Lead Bromide
Abstract
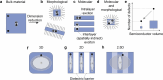
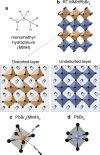
The ability to control materials at the nanoscale has advanced optoelectronic devices, such as LEDs, displays, and quantum light sources. A new frontier is controlling exciton properties beyond quantum size confinement, achieved through single monolayer heterostructures. In the prototypical example of transition metal dichalcogenide heterostructures and moiré superlattices, excitons with long lifetimes, strong binding energies, and tunable dipole moments have been demonstrated and are ideal for optoelectronics and quantum applications. Expanding this material platform is crucial for further progress. This study introduces colloidal nanocrystals (NCs) of monomethylhydrazinium lead bromide (MMHPbBr3), a novel lead halide perovskite (LHP) with a unique "2.5-dimensional" electronic structure. While the spatial dimensionality of the NC extends in all three dimensions, these NCs exhibit excitonic properties intermediate between 2D and 3D LHPs. Density functional theory (DFT) calculations show that MMHPbBr3 features spatially separated electron and hole wave functions, with electrons delocalized in 3D and holes confined in 2D monolayers. Synthesized via a rapid colloidal method, these NCs were characterized by using techniques such as 4D-STEM and nuclear magnetic resonance, confirming their monoclinic structure. Optical analysis revealed size-dependent properties and 3D quantum confinement effects, with three distinct photoluminescence (PL) bands at cryogenic temperatures corresponding to excitons with varying interlayer coupling. PL spectroscopy of single MMHPbBr3 NCs reveals their photon emission statistics, expanding their potential for unconventional quantum material designs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Barré E.; Dandu M.; Kundu S.; Sood A.; da Jornada F. H.; Raja A. Engineering interlayer hybridization in van der Waals bilayers. Nat. Rev. Mater. 2024, 9, 499–508. 10.1038/s41578-024-00666-1. - DOI
-
- Rodin A.; Trushin M.; Carvalho A.; Castro Neto A. H. Collective excitations in 2D materials. Nat. Rev. Phys. 2020, 2, 524–537. 10.1038/s42254-020-0214-4. - DOI
- Li W.; Hadjri Z.; Devenica L. M.; Zhang J.; Liu S.; Hone J.; Watanabe K.; Taniguchi T.; Rubio A.; Srivastava A. Quadrupolar–dipolar excitonic transition in a tunnel-coupled van der Waals heterotrilayer. Nat. Mater. 2023, 22, 1478–1484. 10.1038/s41563-023-01667-1. - DOI - PubMed
-
- Stranks S. D.; Snaith H. J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. 10.1038/nnano.2015.90. - DOI - PubMed
- Akkerman Q. A.; Rainò G.; Kovalenko M. V.; Manna L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. - DOI - PubMed
-
- Akkerman Q. A.; Bladt E.; Petralanda U.; Dang Z.; Sartori E.; Baranov D.; Abdelhady A. L.; Infante I.; Bals S.; Manna L. Fully Inorganic Ruddlesden–Popper Double Cl–I and Triple Cl–Br–I Lead Halide Perovskite Nanocrystals. Chem. Mater. 2019, 31, 2182–2190. 10.1021/acs.chemmater.9b00489. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

