Fusion protein-based COVID-19 vaccines exemplified by a chimeric vaccine based on a single fusion protein (W-PreS-O)
- PMID: 39935478
- PMCID: PMC11811753
- DOI: 10.3389/fimmu.2025.1452814
Fusion protein-based COVID-19 vaccines exemplified by a chimeric vaccine based on a single fusion protein (W-PreS-O)
Abstract
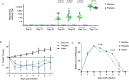
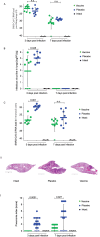
In this article we discuss characteristics of fusion protein-based SARS-CoV-2 vaccines. We focus on recombinant vaccine antigens comprising fusion proteins consisting of combinations of SARS-CoV-2-derived antigens or peptides or combinations of SARS-CoV-2 antigens/peptides with SARS-CoV-2-unrelated proteins/peptides. These fusion proteins are made to increase the immunogenicity of the vaccine antigens and/or to enable special targeting of the immune system. The protein-based vaccine approach is exemplified solely in a proof of concept study by using W-PreS-O, a chimeric vaccine based on a single fusion protein (W-PreS-O), combining RBDs from Wuhan hu-1 wild-type and Omicron BA.1 with the hepatitis B virus (HBV)-derived PreS surface antigen adsorbed to aluminum hydroxide. The W-PreS-O vaccine was evaluated in Syrian hamsters which were immunized three times at three-week intervals with W-PreS-O or with aluminum hydroxide (placebo) before they were infected with Omicron BA.1. Neutralizing antibody (nAB) titers, weight, lung symptoms, and viral loads, as measured using RT-PCR in the upper and lower respiratory tracts, were determined. In addition, infectious virus titers from the lungs were measured using a plaque-forming assay. We found that W-PreS-O-vaccinated hamsters developed robust nABs against Omicron BA.1, showed almost no development of pneumonia, and had significantly reduced infectious virus titers in the lungs. Importantly, the viral loads in the nasal cavities of W-PreS-O-vaccinated hamsters were close to or above the PCR cycle threshold considered to be non-infectious. The data of our proof-of-concept study provides compelling evidence that the W-PreS-O vaccine has protective effect against Omicron BA.1 in a Syrian hamster in vivo infection model and thus support the promising results obtained also for other fusion protein-based SARS-CoV-2 vaccines.
Keywords: COVID-19; SARS-CoV-2; Syrian hamster; fusion protein-based vaccine; infection model; neutralizing antibodies; omicron; vaccine.
Copyright © 2025 Gattinger, Kozlovskaya, Lunin, Gancharova, Sirazova, Apolokhov, Chekina, Gordeychuk, Karaulov, Valenta and Ishmukhametov.
Conflict of interest statement
RV has received research grants from HVD Life-Sciences, Vienna, Austria, WORG Pharmaceuticals, Hangzhou, China and from Viravaxx AG, Vienna, Austria. He serves as consultant for Viravaxx AG. RV and PG are authors on a patent application regarding the vaccine. The authors with Russian affiliation declare that they have prepared the article in their “personal capacity” and/or that they are employed at an academic/research institution where research or education is the primary function of the entity. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. (2022) 399:1618–24. doi: 10.1016/S0140-6736(22)00327-0 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

