Long-term efficacy and safety of alemtuzumab in participants with highly active MS: TOPAZ clinical trial and interim analysis of TREAT-MS real-world study
- PMID: 39935588
- PMCID: PMC11811979
- DOI: 10.1177/17562864241306575
Long-term efficacy and safety of alemtuzumab in participants with highly active MS: TOPAZ clinical trial and interim analysis of TREAT-MS real-world study
Abstract
Background: Alemtuzumab is a disease-modifying therapy for highly active relapsing-remitting multiple sclerosis (RRMS). Sustained efficacy up to 9 years was observed in the phase IIIb/IV open-label TOPAZ clinical trial and assessed in the real-world retrospective and prospective study, TREAT-MS.
Objectives: To examine long-term efficacy and safety of alemtuzumab in participants with multiple sclerosis (MS) and highly active disease (HAD) by combining up to 13 years of TOPAZ data and TREAT-MS interim data.
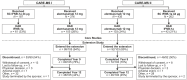
Design: TOPAZ: Randomized participants completing core CARE-MS I and II could receive additional alemtuzumab (12 mg/day, 3 consecutive days; ⩾12 months apart) for 11-13 years after initiating treatment. TREAT-MS: Participants from German MS clinics were observed for 4 years after last alemtuzumab treatment phase.
Methods: Efficacy outcomes (annualized relapse rate (ARR), change in Expanded Disability Status Scale (EDSS), 6-month confirmed disability worsening/improvement, magnetic resonance imaging), and adverse events (AEs) were examined. Primary HAD definition (⩾2 relapses in the year prior to baseline and ⩾1 gadolinium-enhancing lesion at baseline), and two alternative HAD definitions were assessed.
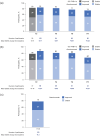
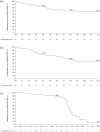
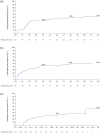
Results: More participants from CARE-MS I (28%) and II (24%) met primary HAD criteria than TREAT-MS (~14%). Mean ARR for alemtuzumab-treated HAD participants was significantly reduced in CARE-MS I and II (0.14 and 0.15, respectively, Years 3-13) and in TREAT-MS (0.24, >2 years). Stable/improved EDSS scores were achieved by 74% of HAD participants in CARE-MS I, 67% in CARE-MS II (both Year 11), and 79% in TREAT-MS (Year 3.6), with 6-month CDI achieved by about half at Year 11 (CARE-MS I, II). Annual treatment-emergent AE incidences declined in TOPAZ and were lower in TREAT-MS.
Conclusion: Sustained efficacy of alemtuzumab was observed for clinical and radiological outcomes in participants with HAD in the TOPAZ clinical trial and real-world TREAT-MS study with no new safety signals.
Trial registration: ClinicalTrials.gov (CARE-MS I, NCT00530348; CARE-MS II, NCT00548405; CARE-MS Extension Study, NCT00930553; TOPAZ, NCT02255656). Paul-Ehrlich-Institut (TREAT-MS, NIS 281).
Keywords: CARE-MS; RRMS; TOPAZ; TREAT-MS; alemtuzumab; highly active disease; multiple sclerosis.
© The Author(s), 2025.
Figures







References
-
- Rush C, MacLean H, Freedman M. Aggressive multiple sclerosis: proposed definition and treatment algorithm. Nat Rev Neurol 2015; 11: 379–389. - PubMed
-
- Díaz C, Zarco L, Rivera D. Highly active multiple sclerosis: an update. Mult Scler Relat Disord 2019; 30: 215–224. - PubMed
-
- Rae-Grant A, Day GS, Marrie RA, et al.. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. - PubMed
-
- Sanofi Belgium. LEMTRADA summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar... (2018, accessed 27 May 2022).
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

