Impact of corticosteroids on the efficacy of first-line pembrolizumab plus chemotherapy in patients with advanced non-small-cell lung cancer
- PMID: 39935765
- PMCID: PMC11811968
- DOI: 10.1177/17588359251318160
Impact of corticosteroids on the efficacy of first-line pembrolizumab plus chemotherapy in patients with advanced non-small-cell lung cancer
Abstract
Background: Systemic corticosteroids (SCs) are associated with reduced survival in patients with advanced non-small-cell lung cancer (NSCLC) receiving immune checkpoint inhibitor (ICI) monotherapy. However, the current first-line standard of care usually involves combined chemotherapy (CT) and ICIs, and the effect of SCs on survival under combined CT and ICI has never been studied.
Objectives: To investigate the association between SC therapy and survival under CT-ICI in advanced-stage NSCLC patients.
Design: We performed a multicenter retrospective cohort study of all advanced-stage NSCLC patients receiving first-line CT-ICI.
Methods: The primary endpoint was progression-free survival (PFS) according to SC exposure status (⩾10 mg/day), adjusted in a multivariate Cox model for the following confounders: age, performance status, hospital admission prior to treatment, number of metastatic sites, brain metastases, bone metastases, PD-L1 status, and histological subtype. Multivariate analyses also explored the association between dosage and SC exposure duration and PFS.
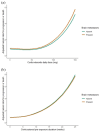
Results: Of the 193 included patients, 43 (22.3%) were receiving SCs, mainly because of symptomatic brain metastases (in 25/43 cases, 58%). In multivariate analysis, SC therapy at a 10 mg/day threshold was not associated with PFS (hazard ratio (HR) = 1.25, 95% confidence interval (CI) 0.77-2.03, p = 0.35). However, SC dose was negatively associated with PFS (HR = 1.08 per 10 mg/day increment, 95% CI 1.01-1.16, p = 0.01) especially at doses ⩾60 mg/day (HR = 3.27 per 10 mg/day increment, 95% CI 2.01-5.35, p < 0.001). Duration of SC therapy was not associated with PFS (HR = 0.97, 95% CI 0.81-1.15, p = 0.71), but SC therapy ⩾4 weeks prior to CT-ICI was associated with shorter PFS (HR = 1.07, 95% CI: 1.01-1.14, p = 0.028).
Conclusion: In this group of patients receiving first-line CT-ICI for advanced NSCLC, SCs at ⩾60 mg/day were associated with shorter PFS, but lower doses were not. Prolonged SC therapy prior to CT-ICI was associated with shorter PFS. Larger studies are required to confirm these results.
Keywords: chemotherapy-immunotherapy combination; non-small-cell lung cancer; pembrolizumab; systemic steroids.
© The Author(s), 2025.
Conflict of interest statement
G.P., A.R., A.T., and S.B. declare no conflict of interest. E.W. declares participation in Takeda boards. C.G. declares compensation for interventions with Pfizer and Boehringer Ingelheim. A.C. discloses the following conflicts of interest: grants from Abbvie, consulting fees from Novartis, F. Hoffman La Roche, Pierre Fabre, Pfizer, Exeliom, Abbvie, Sanofi, Janssen, honoraria for lectures or presentations from Astra Zeneca, MSD, Pfizer, Novartis, Takeda, Janssen, Roche, Abbvie, Amgen, support for attending meetings from Pfizer, MSD, Novartis, Daiichi, Astra-Zeneca, and participation in a Data Safety Monitoring or Advisory Board for InhaTarget. A.S. declares grants and research support from AS, involvement as an investigator in phase I, II, and III clinical trials sponsored by Amphera, Astra-Zeneca, BMS, MSD, Novartis, Regeneron, Roche, Trizell, with no personal remuneration, all honoraria being received by his institution (CHU de Lille, France), honoraria for participation in scientific or advisory boards from Astra-Zeneca, BMS, Leo Pharma, MSD, Roche, and Sanofi, and invitations to international oncology meetings (ASCO, ESMO, WCLC) funded by Amgen, Astra-Zeneca, BMS, MSD, and Roche.
Figures


References
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al.. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378(22): 2078–2092. - PubMed
-
- Paz-Ares L, Luft A, Vicente D, et al.. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379(21): 2040–2051. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al.. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375(19): 1823–1833. - PubMed
-
- Libert C, Dejager L. How steroids steer T cells. Cell Rep 2014; 7(4): 938–939. - PubMed
-
- Bereshchenko O, Coppo M, Bruscoli S, et al.. GILZ promotes production of peripherally induced Treg cells and mediates the crosstalk between glucocorticoids and TGF-β signaling. Cell Rep 2014; 7(2): 464–475. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

