Synthetic embryology of the human heart
- PMID: 39935786
- PMCID: PMC11810959
- DOI: 10.3389/fcell.2024.1478549
Synthetic embryology of the human heart
Abstract
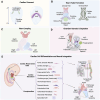
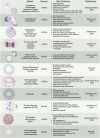
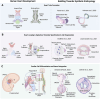
The evolution of stem cell-based heart models from cells and tissues to organoids and assembloids and recently synthetic embryology gastruloids, is poised to revolutionize our understanding of cardiac development, congenital to adult diseases, and patient customized therapies. Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have already been integrated into transplantable patches and are in preclinical efforts to reverse fibrotic scarring from myocardial infarctions. To inform on the complexity of heart diseases, multi-tissue morphogenic heart models are needed that replicate fundamental components of heart function to heart organogenesis in vitro and which require a deep understanding of heart development. Organoid and assembloid models capture selected multicellular cardiac processes, such as chamber formation and priming events for vascularization. Gastruloid heart models offer deeper insights as synthetic embryology to mimic multi-staged developmental events of in vivo heart organogenesis including established heart fields, crescent formation and heart tube development along with vascular systemic foundation and even further steps. The human Elongating Multi-Lineage Organized Cardiac (EMLOC) gastruloid model captures these stages and additional events including chamber genesis, patterned vascularization, and extrinsic central and intrinsic cardiac nervous system (CNS-ICNS) integration guided by spatiotemporal and morphogenic processes with neural crest cells. Gastruloid synthetic embryology heart models offer new insights into previously hidden processes of development and provide powerful platforms for addressing heart disease that extends beyond cardiomyocytes, such as arrhythmogenic diseases, congenital defects, and systemic injury interactions, as in spinal cord injuries. The holistic view that is emerging will reveal heart development and disease in unprecedented detail to drive transformative state-of-the-art innovative applications for heart health.
Keywords: ICNS; assembloid; cardiogenesis; gastruloid; hiPSC; neurons; organogenesis; organoid.
Copyright © 2025 Paredes-Espinosa and Paluh.
Conflict of interest statement
The research was conducted as part of the aforementioned CATN2 award between the Paluh lab and Cytocybernetics, Inc a New York business with expertise that includes cardiac electrophysiology.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

