Lipid Nanoparticle Delivery System for Normalization of Tumor Microenvironment and Tumor Vascular Structure
- PMID: 39935791
- PMCID: PMC11811622
- DOI: 10.34133/bmr.0144
Lipid Nanoparticle Delivery System for Normalization of Tumor Microenvironment and Tumor Vascular Structure
Abstract
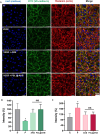
Tumors grow by receiving oxygen and nutrients from the surrounding blood vessels, leading to rapid angiogenesis. This results in functionally and structurally abnormal vasculature characterized by high permeability and irregular blood flow, causing hypoxia within the tumor microenvironment (TME). Hypoxia exacerbates the secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), further perpetuating abnormal vessel formation. This environment compromises the efficacy of radiotherapy, immunotherapy, and chemotherapy. In this study, we developed a pH-sensitive liposome (PSL) system, termed OD_PSL@AKB, to co-deliver oxygen (OD) and razuprotafib (AKB-9778) to tumors. This system rapidly responds to the acidic TME to alleviate hypoxia and inhibit VEGF secretion, restoring VE-cadherin expression in hypoxic endothelial cell/cancer cell cocultures. Our findings highlight the potential of OD_PSL@AKB in normalizing tumor vasculature and improving therapeutic efficacy.
Copyright © 2025 Heejin Ha et al.
Conflict of interest statement
Competing interests: J.C. is the CEO/founder and Y.C. is the CTO of Feynman Institute of Technology at the Nanomedicine Corporation.
Figures







References
-
- Lee W, Yoon B, Lee J, Jung S, Oh YS, Ko J, Jeon NL. Machine learning-aided three-dimensional morphological quantification of angiogenic vasculature in the multiculture microfluidic platform. Biochip J. 2023;17:357–368.
LinkOut - more resources
Full Text Sources

