Mechanisms and roles of membrane-anchored ATG8s
- PMID: 39936034
- PMCID: PMC11810923
- DOI: 10.3389/fcell.2025.1532050
Mechanisms and roles of membrane-anchored ATG8s
Abstract
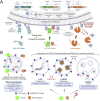
Autophagy-related protein 8 (ATG8) family proteins, including LC3 and GABARAP subfamilies, are pivotal in canonical autophagy, driving autophagosome formation, cargo selection, and lysosomal fusion. However, recent studies have identified non-canonical roles for lipidated ATG8 in processes such as LC3-associated phagocytosis (LAP), LC3-associated endocytosis (LANDO), and lipidated ATG8-mediated secretory autophagy. These pathways expand ATG8's functional repertoire in immune regulation, membrane repair, and pathogen clearance, as ATG8 becomes conjugated to single-membrane structures (e.g., phagosomes and lysosomes). This review examines the molecular mechanisms of ATG8 lipidation, focusing on its selective conjugation to phosphatidylethanolamine (PE) in autophagy and phosphatidylserine (PS) in CASM. We highlight LIR-based probes and LC3/GABARAP-specific deconjugases as critical tools that allow precise tracking and manipulation of ATG8 in autophagic and non-autophagic contexts. These advancements hold therapeutic promise for treating autophagy-related diseases, including cancer and neurodegenerative disorders, by targeting ATG8-driven pathways that maintain cellular homeostasis.
Keywords: LAP; LC3/GABARAP; LIR motif; Lando; autophagy; deconjugase; non-canonical autophagy; probe.
Copyright © 2025 Lee, Park, Jang and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alemu E. A., Lamark T., Torgersen K. M., Birgisdottir A. B., Larsen K. B., Jain A., et al. (2012). ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 287 (47), 39275–39290. 10.1074/jbc.M112.378109 - DOI - PMC - PubMed
-
- Birgisdottir Å. B., Mouilleron S., Bhujabal Z., Wirth M., Sjøttem E., Evjen G., et al. (2019). Members of the autophagy class III phosphatidylinositol 3-kinase complex I interact with GABARAP and GABARAPL1 via LIR motifs. Autophagy 15 (8), 1333–1355. 10.1080/15548627.2019.1581009 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

