Remote cognitive tests predict neurodegenerative biomarkers in the Insight 46 cohort
- PMID: 39936232
- PMCID: PMC11815243
- DOI: 10.1002/alz.14572
Remote cognitive tests predict neurodegenerative biomarkers in the Insight 46 cohort
Erratum in
-
Correction to "Remote cognitive tests predict neurodegenerative biomarkers in the Insight 46 cohort".Alzheimers Dement. 2025 Apr;21(4):e70202. doi: 10.1002/alz.70202. Alzheimers Dement. 2025. PMID: 40289705 Free PMC article. No abstract available.
Abstract
Background: Alzheimer's disease-related biomarkers detect pathology years before symptoms emerge, when disease-modifying therapies might be most beneficial. Remote cognitive testing provides a means of assessing early cognitive changes. We explored the relationship between neurodegenerative biomarkers and cognition in cognitively normal individuals.
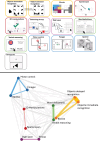
Methods: We remotely deployed 13 computerized Cognitron tasks in 255 Insight 46 participants. We generated amyloid load and positivity, white matter hyperintensity volume (WMHV), whole brain and hippocampal volumes at age 73, plus rates of change over 2 years. We examined the relationship between Cognitron, biomarkers, and standard neuropsychological tests.
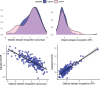
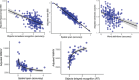
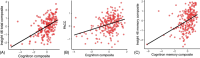
Results: Slower response time on a delayed recognition task predicted amyloid positivity (odds ratio [OR] = 1.79, confidence interval [CI]: 1.15, 2.95), and WMHV (1.23, CI: 1.00, 1.56). Brain and hippocampal atrophy rates correlated with poorer visuospatial performance (b = -0.42, CI: -0.80, -0.05) and accuracy on immediate recognition (b = -0.01, CI: -0.012, -0.001), respectively. Standard tests correlated with Cognitron composites (rho = 0.50, p < 0.001).
Discussion: Remote computerized testing correlates with standard supervised assessments and holds potential for studying early cognitive changes associated with neurodegeneration.
Highlights: 70% of the Online 46 cohort performed a set of remote online cognitive tasks. Response time and accuracy on a memory task predicted amyloid status and load (SUVR). Accuracy on memory and spatial span tasks correlated with longitudinal atrophy rate. The Cognitron tasks correlated with standard supervised cognitive tests. Online cognitive testing can help identify early AD-related memory deficits.
Keywords: Alzheimer's disease; Insight 46; amyloid; biomarkers; computerized cognitive testing; dementia; memory; neurodegeneration.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
A.H. is owner/director of H2 Cognitive Designs Ltd and Future Cognition Ltd, which produce online assessment technology and provide online survey data collection for third parties. P.H. is founder and director of H2 Cognitive Designs LTD, which develops and markets online cognitive tests. W.T. is an employee of H2 Cognitive Designs LTD. PM is lead for an NIHR‐funded trial with drug/placebo provided by Takeda Pharmaceuticals and sits on the Data Monitoring Committee for a trial being carried out by Johnson and Johnson. P.M. is vice chair of the Alzheimer's Society Research Strategy Council, and NIHR Specialty Lead for Dementia and Neurodegeneration, Research Delivery Network. He is also an independent member of a data monitoring committee. C.S. is a scientific BrainKey scientific advisor, part of MONAI advisory board. D.C. is chair of the Alzheimer's Association Neuroimaging professorial interest area and a member of the Scientific Programming Committee of the Alzheimer's Association. He also reports grants from the National Institute of Health. J.B. is an NFRFT 2024 Application Stage Expert Panel, director of SENBOX and Chair of Faculty Research Degrees Committee at University College London. J.M.S. is the Chief Medical Officer of Alzheimer's Research UK and clinical advisor at the UK Dementia Research Institute. He reports grants from the NIHR, LifeArc Foundation, and the British Hearth Foundation.
Figures

References
-
- De Strooper B, Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2016;164(4):603‐615. - PubMed
-
- Jia J, Ning Y, Chen M, et al. Biomarker Changes during 20 Years Preceding Alzheimer's Disease. N Engl J Med. 2024;390(8):712‐722. - PubMed
-
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in Early Alzheimer's Disease. N Engl J Med. 2021;384(18):1691‐1704. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023;388(1):9‐21. - PubMed
MeSH terms
Substances
Grants and funding
- CSUB19166/Dementias Platform UK
- PR/ylr/18575/Wolfson Foundation
- UCC14191/Brain Research Trust
- MC_UU_00019/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_10019/3/MRC_/Medical Research Council/United Kingdom
- MR/W00710X/1/MRC_/Medical Research Council/United Kingdom
- National Institute for Health and Care Research (NIHR)
- ARUK-PG2014-1946/Alzheimer's Research UK
- ARUK-PG2017-1946/Alzheimer's Research UK
- Football Association
- SG-666374-UKBIRTHCOHORT/ALZ/Alzheimer's Association/United States
- NIHR Imperial Biomedical Research Centre
- AS-17-JF-011/ALZS_/Alzheimer's Society/United Kingdom
- UCLH/UCL Biomedical Research Centre (UCLH Biomedical Research Centre)
- Dementia Platforms UK
- The British Heart Foundation
- Lifearc Foundation
LinkOut - more resources
Full Text Sources
Medical

