The Dementia SomaSignal Test (dSST): A plasma proteomic predictor of 20-year dementia risk
- PMID: 39936291
- PMCID: PMC11851157
- DOI: 10.1002/alz.14549
The Dementia SomaSignal Test (dSST): A plasma proteomic predictor of 20-year dementia risk
Abstract
Introduction: There is an unmet need for tools to quantify dementia risk during its multi-decade preclinical/prodromal phase, given that current biomarkers predict risk over shorter follow-up periods and are specific to Alzheimer's disease.
Methods: Using high-throughput proteomic assays and machine learning techniques in the Atherosclerosis Risk in Communities study (n = 11,277), we developed the Dementia SomaSignal Test (dSST).
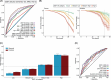
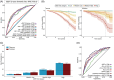
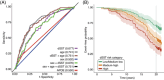
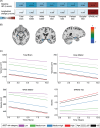
Results: In addition to outperforming existing plasma biomarkers, the dSST predicted mid-life dementia risk over a 20-year follow-up across two independent cohorts with different ethnic backgrounds (areas under the curve [AUCs]: dSST 0.68-0.70, dSST+age 0.75-0.81). In a separate cohort, the dSST was associated with longitudinal declines across multiple cognitive domains, accelerated brain atrophy, and elevated measures of neuropathology (as evidenced by positron emission tomography and plasma biomarkers).
Discussion: The dSST is a cost-effective, scalable, and minimally invasive protein-based prognostic aid that can quantify risk up to two decades before dementia onset.
Highlights: The Dementia SomaSignal Test (dSST) predicts 20-year dementia risk across two independent cohorts. dSST outperforms existing plasma biomarkers in predicting multi-decade dementia risk. dSST predicts cognitive decline and accelerated brain atrophy in a third cohort. dSST is a prognostic aid that can predict dementia risk over two decades.
Keywords: dementia; machine learning; prognosis; proteomics.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
C.P., K.L., M.S., H.B., and S.A.W. are current or former employees of SomaLogic Operating Co., Inc, and/or Standard BioTools. N.K., M.S., S.K., M.F., and I.W. are current employees of NEC Solution Innovators Limited and/or FonesLife Corporation. The remaining authors declare no conflicts of interest. Author disclosures are available in the Supporting Information.
Figures



References
MeSH terms
Substances
Grants and funding
- U01HL096812/NHLBI, NIA, NINDS, NIDCD
- 75N92022D00002/HL/NHLBI NIH HHS/United States
- U01 HL096917/HL/NHLBI NIH HHS/United States
- U01 HL096902/HL/NHLBI NIH HHS/United States
- U01HL096902/NHLBI, NIA, NINDS, NIDCD
- 75N92022D00004/HL/NHLBI NIH HHS/United States
- U01HL096917/NHLBI, NIA, NINDS, NIDCD
- U01HL096814/NHLBI, NIA, NINDS, NIDCD
- U01 HL096814/HL/NHLBI NIH HHS/United States
- 75N92022D00003/HL/NHLBI NIH HHS/United States
- 75N92022D00005/HL/NHLBI NIH HHS/United States
- Intramural Research Program (IRP) of the National Institute on Aging (NIA)
- 75N92022D00001/HL/NHLBI NIH HHS/United States
- National Center for Geriatrics and Gerontology
- P30 AG066507/AG/NIA NIH HHS/United States
- U01 HL096812/HL/NHLBI NIH HHS/United States
- Nagoya University
- U01HL096899/NHLBI, NIA, NINDS, NIDCD
- NEC Solution Innovators Limited
- U01 HL096899/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

