Electrodiagnostic Assessment of Peri-Procedural Iatrogenic Peripheral Nerve Injuries and Rehabilitation
- PMID: 39936306
- PMCID: PMC11998969
- DOI: 10.1002/mus.28364
Electrodiagnostic Assessment of Peri-Procedural Iatrogenic Peripheral Nerve Injuries and Rehabilitation
Abstract
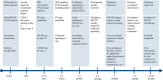
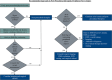
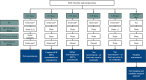
Iatrogenic nerve injuries are a significant concern for medical professionals and the patients affected. Peri-procedural nerve injuries result in functional deficits associated with pain and disability. The exact pathophysiology and etiology of peri-procedural nerve injuries are complex and often elude providers. The rates of injury to specific nerves are unclear and relate to both procedural and patient specific risk factors. Initial classification of the nerve injury into neurapraxia, axonotmesis, mixed nerve injury, or possible complete transection (neurotmesis) guides rehabilitation and management. Electrodiagnostic medical consultation at least four weeks post-injury, supplemented with nerve imaging (ultrasound and magnetic resonance imaging), can allow for accurate nerve injury classification. Supplemented with nerve imaging and detailed clinical evaluation, treatment, recovery and rehabilitation can be maximized. Recognizing nerves at risk associated with medical and surgical procedures can facilitate injury avoidance and early diagnosis. If a nerve injury is incomplete, in an optimized physiologic milieu (good glucose control, smoking cessation, etc.), there is a good potential for spontaneous (total or partial) improvement over time. Surgical referral should be considered for severe nerve injuries within 6 months, especially if there is concern for neurotmesis, and/or deteriorating nerve function. This review gives guidance for approaching peri-procedural peripheral nerve injuries, including the timing and the role of electrodiagnostic medical consultation including serial electrodiagnostic studies in management and rehabilitation.
Keywords: electrodiagnosis; electromyogram; iatrogenic peripheral nerve injury; nerve rehabilitation; perioperative peripheral nerve injury.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Epidemiology of Traumatic Peripheral Nerve Injuries Evaluated with Electrodiagnostic Studies in a Tertiary Care Hospital Clinic.P R Health Sci J. 2016 Jun;35(2):76-80. P R Health Sci J. 2016. PMID: 27232868
-
Assessment, management, and rehabilitation of traumatic peripheral nerve injuries for non-surgeons.Muscle Nerve. 2025 May;71(5):696-714. doi: 10.1002/mus.28185. Epub 2024 Jun 21. Muscle Nerve. 2025. PMID: 39030747 Free PMC article. Review.
-
Electrophysiological and etiological evaluation of 119 cases of wrist drop: A single center study.J Pak Med Assoc. 2019 May;69(5):672-676. J Pak Med Assoc. 2019. PMID: 31105286
-
Peripheral Nerve Injuries: Electrophysiology for the Neurosurgeon.Neurol India. 2019 Nov-Dec;67(6):1419-1422. doi: 10.4103/0028-3886.273626. Neurol India. 2019. PMID: 31857526 Review.
-
PERIPHERAL NERVE INJURY IN SPORTS.Acta Clin Croat. 2018 Sep;57(3):561-569. doi: 10.20471/acc.2018.57.03.20. Acta Clin Croat. 2018. PMID: 31168190 Free PMC article. Review.
References
-
- Moore A. E., Zhang J., and Stringer M. D., “Iatrogenic Nerve Injury in a National no‐Fault Compensation Scheme: An Observational Cohort Study,” International Journal of Clinical Practice 66, no. 4 (2012): 409–416. - PubMed
-
- Carter J. T., Pisquiy J. J., Polmear M., Khalifa R., and Gonzalez G., “A New Classification of Iatrogenic Peripheral Nerve Injuries,” Biologie et Médecine 12, no. 3 (2020): 464.
-
- Staff N. P., Engelstad J., Klein C. J., et al., “Post‐Surgical Inflammatory Neuropathy,” Brain 133, no. 10 (2010): 2866–2880. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

