Nipocalimab, an immunoselective FcRn blocker that lowers IgG and has unique molecular properties
- PMID: 39936406
- PMCID: PMC11834464
- DOI: 10.1080/19420862.2025.2461191
Nipocalimab, an immunoselective FcRn blocker that lowers IgG and has unique molecular properties
Abstract
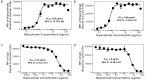
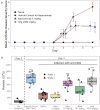
Nipocalimab is a human immunoglobulin G (IgG)1 monoclonal antibody that binds to the neonatal Fc receptor (FcRn) with high specificity and high affinity at both neutral (extracellular) and acidic (intracellular) pH, resulting in the reduction of circulating IgG levels, including those of pathogenic IgG antibodies. Here, we present the molecular, cellular, and nonclinical characteristics of nipocalimab that support the reported clinical pharmacology and potential clinical application in IgG-driven, autoantibody- and alloantibody-mediated diseases. The crystal structure of the nipocalimab antigen binding fragment (Fab)/FcRn complex reveals its binding to a unique epitope on the IgG binding site of FcRn that supports the observed pH-independent high-binding affinity to FcRn. Cell-based and in vivo studies demonstrate concentration/dose- and time-dependent FcRn occupancy and IgG reduction. Nipocalimab selectively reduces circulating IgG levels without detectable effects on other adaptive and innate immune functions. In vitro experiments and in vivo studies in mice and cynomolgus monkeys generated data that align with observations from clinical studies of nipocalimab in IgG autoantibody- and alloantibody-mediated diseases.
Keywords: Adaptive immune functions; FcRn blockers; in vivo models; innate immune functions; molecular properties; pharmacologic properties.
Conflict of interest statement
Figures







References
-
- Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, Mason J, Sattar N, McMurray JJV, McInnes IB, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–1890. doi:10.1016/S0140-6736(23)00457-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
