Dynamical organization of vimentin intermediate filaments in living cells revealed by MoNaLISA nanoscopy
- PMID: 39936518
- PMCID: PMC12127793
- DOI: 10.1042/BSR20241133
Dynamical organization of vimentin intermediate filaments in living cells revealed by MoNaLISA nanoscopy
Abstract
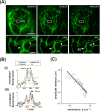
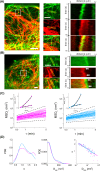
Intermediate filaments are intimately involved in the mechanical behavior of cells. Unfortunately, the resolution of optical microscopy limits our understanding of their organization. Here, we combined nanoscopy, single-filament tracking, and numerical simulations to inspect the dynamical organization of vimentin intermediate filaments in live cells. We show that a higher proportion of peripheral versus perinuclear vimentin pools are constrained in their lateral motion in the seconds time window, probably due to their cross-linking to other cytoskeletal networks. In a longer time scale, active forces become evident and affect similarly both pools of filaments. Our results provide a detailed description of the dynamical organization of the vimentin network in live cells and give some cues on its response to mechanical stimuli.
Keywords: MoNaLISA nanoscopy; cytoskeleton; vimentin filaments.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

