Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management
- PMID: 39936576
- PMCID: PMC11966364
- DOI: 10.1002/ajh.27625
Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management
Abstract
Disease overview: Acute myeloid leukemia (AML) is a bone marrow stem cell cancer that is often fatal despite available treatments. Diagnosis, risk assessment, monitoring, and therapeutic management of AML have changed dramatically in the last decade due to increased pathophysiologic understanding, improved assessment technology, and the addition of at least 12 approved therapies.
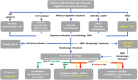
Diagnosis: The diagnosis is based on the presence of immature leukemia cells in the blood, and/or bone marrow or less often in extra-medullary tissues. New biological insights have been integrated into recent classification systems.
Risk assessment: The European Leukemia Network has published risk classification algorithms for both intensively and non-intensively treated patients based on cytogenetic and on molecular findings. Prognostic factors may differ based on the therapeutic approach.
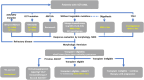
Monitoring: Our increasing ability to quantify lower levels of measurable residual disease (MRD) potentially allows better response assessment, as well as dynamic monitoring of disease status. The incorporation of MRD findings into therapeutic decision-making is rapidly evolving.
Risk adapted therapy: The availability of 12 newly approved agents has been welcomed; however, optimal strategies incorporating newer agents into therapeutic algorithms are debated. The overarching approach integrates patient and caregiver goals of care, comorbidities, and disease characteristics.
Keywords: AML diagnosis; AML therapy; AML‐molecular diagnosis and therapy; measureable residual disease in AML; neoplasia‐myeloid leukemias and dysplasias.
© 2025 The Author(s). American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
R.M.S. reports grants and personal fees from Abbvie, personal fees from Actinium, grants and personal fees from Agios, personal fees from Argenx, grants from Arog, personal fees from Astellas, personal fees from AstraZeneca, personal fees from Biolinerx, personal fees from Celgene, personal fees from Daiichi‐Sankyo, personal fees from Elevate, personal fees from Gemoab, personal fees from Janssen, personal fees from Jazz, personal fees from Macrogenics, grants and personal fees from Novartis, personal fees from Otsuka, personal fees from Pfizer, personal fees from Hoffman LaRoche, personal fees from Stemline, personal fees from Syndax, personal fees from Syntrix, personal fees from Syros, personal fees from Takeda, from Trovagene, outside the submitted work. M.S. reports consulting and personal fees from Curtis Oncology, Haymarket Media, Boston Consulting; Membership on advisory board of Novartis, Kymera. S.S. do not have conflicts of interest to declare.
Figures



References
-
- Dohner H., Weisdorf D. J., and Bloomfield C. D., “Acute Myeloid Leukemia,” New England Journal of Medicine 373, no. 12 (2015): 1136–1152. - PubMed
-
- Juliusson G., Antunovic P., Derolf A., et al., “Age and Acute Myeloid Leukemia: Real World Data on Decision to Treat and Outcomes From the Swedish Acute Leukemia Registry,” Blood 113, no. 18 (2009): 4179–4187. - PubMed
-
- “SEER Data Base: Cancer Stat Facts: Leukemia” — Acute Myeloid Leukemia (AML), accessed June 11, 2023, https://seer.cancer.gov/statfacts/html/amyl.html.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

