Glucokinase activity controls peripherally located subpopulations of β-cells that lead islet Ca2+ oscillations
- PMID: 39936635
- PMCID: PMC11820133
- DOI: 10.7554/eLife.103068
Glucokinase activity controls peripherally located subpopulations of β-cells that lead islet Ca2+ oscillations
Abstract
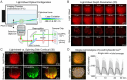
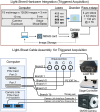
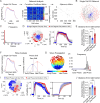
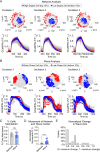
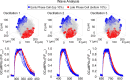
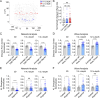
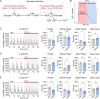
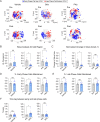
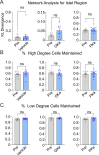
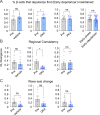
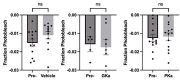
Oscillations in insulin secretion, driven by islet Ca2+ waves, are crucial for glycemic control. Prior studies, performed with single-plane imaging, suggest that subpopulations of electrically coupled β-cells have privileged roles in leading and coordinating the propagation of Ca2+ waves. Here, we used three-dimensional (3D) light-sheet imaging to analyze the location and Ca2+ activity of single β-cells within the entire islet at >2 Hz. In contrast with single-plane studies, 3D network analysis indicates that the most highly synchronized β-cells are located at the islet center, and remain regionally but not cellularly stable between oscillations. This subpopulation, which includes 'hub cells', is insensitive to changes in fuel metabolism induced by glucokinase and pyruvate kinase activation. β-Cells that initiate the Ca2+ wave (leaders) are located at the islet periphery, and strikingly, change their identity over time via rotations in the wave axis. Glucokinase activation, which increased oscillation period, reinforced leader cells and stabilized the wave axis. Pyruvate kinase activation, despite increasing oscillation frequency, had no effect on leader cells, indicating the wave origin is patterned by fuel input. These findings emphasize the stochastic nature of the β-cell subpopulations that control Ca2+ oscillations and identify a role for glucokinase in spatially patterning 'leader' β-cells.
Keywords: calcium oscillation; cell biology; glucokinase; light-sheet microscope; mouse; pancreatic islets; β-cell.
© 2024, Jin, Briggs et al.
Conflict of interest statement
EJ, JB, RB, MM No competing interests declared
Figures



Update of
-
Glucokinase activity controls peripherally-located subpopulations of β-cells that lead islet Ca2+ oscillations.bioRxiv [Preprint]. 2024 Dec 11:2024.08.21.608680. doi: 10.1101/2024.08.21.608680. bioRxiv. 2024. Update in: Elife. 2025 Feb 12;13:RP103068. doi: 10.7554/eLife.103068. PMID: 39229244 Free PMC article. Updated. Preprint.
Similar articles
-
Opportunity makes a hub or a leader.Elife. 2025 Feb 12;14:e105929. doi: 10.7554/eLife.105929. Elife. 2025. PMID: 39936640 Free PMC article.
-
Glucokinase activity controls peripherally-located subpopulations of β-cells that lead islet Ca2+ oscillations.bioRxiv [Preprint]. 2024 Dec 11:2024.08.21.608680. doi: 10.1101/2024.08.21.608680. bioRxiv. 2024. Update in: Elife. 2025 Feb 12;13:RP103068. doi: 10.7554/eLife.103068. PMID: 39229244 Free PMC article. Updated. Preprint.
-
Phosphofructo-2-kinase/fructose-2,6-bisphosphatase modulates oscillations of pancreatic islet metabolism.PLoS One. 2012;7(4):e34036. doi: 10.1371/journal.pone.0034036. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22532827 Free PMC article.
-
Newer perspective on the coupling between glucose-mediated signaling and β-cell functionality.Endocr J. 2020 Jan 28;67(1):1-8. doi: 10.1507/endocrj.EJ19-0335. Epub 2019 Nov 6. Endocr J. 2020. PMID: 31694991 Review.
-
Metabolic regulation of calcium signaling in beta cells.Semin Cell Dev Biol. 2020 Jul;103:20-30. doi: 10.1016/j.semcdb.2020.01.008. Epub 2020 Feb 19. Semin Cell Dev Biol. 2020. PMID: 32085965 Review.
Cited by
-
CRISP: Correlation-Refined Image Segmentation Process.bioRxiv [Preprint]. 2024 Oct 4:2024.08.23.609461. doi: 10.1101/2024.08.23.609461. bioRxiv. 2024. Update in: BMC Bioinformatics. 2025 May 26;26(1):135. doi: 10.1186/s12859-025-06150-z. PMID: 39253465 Free PMC article. Updated. Preprint.
-
Opportunity makes a hub or a leader.Elife. 2025 Feb 12;14:e105929. doi: 10.7554/eLife.105929. Elife. 2025. PMID: 39936640 Free PMC article.
-
CRISP: correlation-refined image segmentation process.BMC Bioinformatics. 2025 May 26;26(1):135. doi: 10.1186/s12859-025-06150-z. BMC Bioinformatics. 2025. PMID: 40419943 Free PMC article.
References
-
- Adams MT, Dwulet JM, Briggs JK, Reissaus CA, Jin E, Szulczewski JM, Lyman MR, Sdao SM, Kravets V, Nimkulrat SD, Ponik SM, Merrins MJ, Mirmira RG, Linnemann AK, Benninger RK, Blum B. Reduced synchroneity of intra-islet Ca2+ oscillations in vivo in Robo-deficient β cells. eLife. 2021;10:e61308. doi: 10.7554/eLife.61308. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01DK140904/DK/NIDDK NIH HHS/United States
- R01 DK102950/DK/NIDDK NIH HHS/United States
- R01 DK113103/DK/NIDDK NIH HHS/United States
- R01DK113103/DK/NIDDK NIH HHS/United States
- I01 BX005113/BX/BLRD VA/United States
- P30 DK116073/DK/NIDDK NIH HHS/United States
- DGE-1938058_Briggs/National Science Foundation
- I01BX005113/Biomedical Laboratory Research and Development, VA Office of Research and Development
- R01DK102950/DK/NIDDK NIH HHS/United States
- R01DK106412/DK/NIDDK NIH HHS/United States
- R01 DK140904/DK/NIDDK NIH HHS/United States
- R01DK127637/DK/NIDDK NIH HHS/United States
- R01 DK127637/DK/NIDDK NIH HHS/United States
- R01 DK106412/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

