RyR1 Is Involved in the Control of Myogenesis
- PMID: 39936950
- PMCID: PMC11817019
- DOI: 10.3390/cells14030158
RyR1 Is Involved in the Control of Myogenesis
Abstract
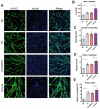
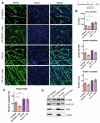
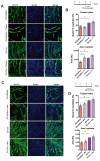
The RyR1 calcium release channel is a key player in skeletal muscle excitation-contraction coupling. Mutations in the RYR1 gene are associated with congenital myopathies. Recently, a role of RyR1 in myotubes differentiation has been proposed and attributed to its calcium channel function, which nonetheless remains to be clearly demonstrated. In order to clarify RyR1 role in myogenesis, we have developed an in vitro model, the so-called RyR1-Rec myotubes, which are mouse primary myotubes with an inducible decrease in RyR1 protein amount and in RyR1-mediated calcium release. Using this model, we showed that the RyR1 protein decrease was responsible for an increase in both differentiation and fusion, from the RNA level to the morphological level, without affecting the myogenic factors MyoD and MyoG. Although an increase in mTOR pathway was observed in RyR1-Rec myotubes, it did not seem to be responsible for the role of RyR1 in myogenesis. Additionally, even if modulation of intracellular calcium level affected RyR1-Rec myotubes differentiation, we have shown that the role of RyR1 in myogenesis was independent of its calcium channel function. Therefore, our findings indicate that, besides its pivotal role as a calcium channel responsible for muscle contraction, RyR1 fulfills a calcium-independent inhibitor function of myogenesis.
Keywords: RyR1; calcium; myogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lawal T.A., Todd J.J., Witherspoon J.W., Bönnemann C.G., Dowling J.J., Hamilton S.L., Meilleur K.G., Dirksen R.T. Ryanodine receptor 1-related disorders: An historical perspective and proposal for a unified nomenclature. Skelet. Muscle. 2020;10:32. doi: 10.1186/s13395-020-00243-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

