Neutralizing IL-15 Inhibits Tissue-Damaging Immune Response in Ex Vivo Cultured Untreated Celiac Intestinal Mucosa
- PMID: 39937025
- PMCID: PMC11818035
- DOI: 10.3390/cells14030234
Neutralizing IL-15 Inhibits Tissue-Damaging Immune Response in Ex Vivo Cultured Untreated Celiac Intestinal Mucosa
Abstract
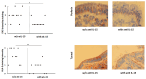
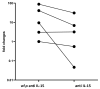
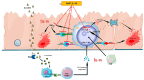
In celiac disease (CeD), interleukin 15 (IL-15) affects the epithelial barrier by acting on intraepithelial lymphocytes, promoting interferon γ (IFN-γ) production and inducing strong cytotoxic activity as well as eliciting apoptotic death of enterocytes by the Fas/Fas ligand system. This study investigates the effects of a monoclonal antibody neutralizing the effects of IL-15 (aIL-15) on tissue-damaging immune response in untreated CeD patients by using an organ culture system. Jejunal biopsies from 10 untreated CeD patients were cultured ex vivo with or without aIL-15. Epithelial expressions of CD95/Fas, HLA-E and perforin were analyzed by immunohistochemistry. Apoptosis was detected in the epithelium by using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay. Additionally, the surface epithelium compartment of ex vivo cultured biopsy samples was isolated by laser capture microdissection (LCM). RNA from each LCM sample was extracted and the relative expression of IFN-γ was evaluated by quantitative reverse transcriptase-PCR (qRT-PCR). Biopsies cultured with the aIL-15 antibody showed a reduction in Fas, HLA-E and perforin epithelial expression, as well as a decrease in epithelial TUNEL+ cells compared to biopsies cultured without the aIL-15 antibody. Moreover, downregulation of epithelial IFN-γ expression was recorded in biopsies incubated with aIL-15, compared to those cultured without aIL-15. Our findings suggest that neutralizing the effects of IL-15 in ex vivo cultured untreated CeD intestinal mucosa could block apoptosis by downregulating Fas and HLA-E expression and the release of cytotoxic proteins, such as perforin. Furthermore, it can dampen the hyperactive immune response by reducing IFN-γ expression. More generally, our study provides new evidence for the effects of anti-IL-15 neutralizing monoclonal antibodies in preventing or repairing epithelial damage and further supports the concept that IL-15 is a meaningful therapeutic target in CeD, or inflammatory diseases associated with the upregulation of IL-15.
Keywords: IL-15; celiac disease; cytotoxicity; therapy.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

