Meningeal leukaemic aggregates as foci of cell expansion and chemoresistance in acute lymphoblastic leukaemia metastasis
- PMID: 39937211
- PMCID: PMC12119772
- DOI: 10.1007/s13402-025-01043-y
Meningeal leukaemic aggregates as foci of cell expansion and chemoresistance in acute lymphoblastic leukaemia metastasis
Abstract
Purpose: Central nervous system (CNS) involvement and/or relapse remains one of the most important causes of morbidity/mortality in paediatric B-cell precursor acute lymphoblastic leukaemia (BCP-ALL) patients. To identify novel therapeutic targets and develop less aggressive therapies, a better understanding of the cellular and molecular microenvironment in leptomeningeal metastases is key. Here, we aimed to investigate the formation of metastatic leptomeningeal aggregates and their relevance to the expansion, survival and chemoresistance acquisition of leukaemia cells.
Methods: We used BCP-ALL xenograft mouse models, combined with immunohistofluorescence and flow cytometry, to study the development of CNS metastasis and the contribution of leptomeningeal cells to the organisation of leukaemic aggregates. To in vitro mimic the CNS metastasis, we established co-cultures of three-dimensional (3D) ALL cell spheroids and human leptomeningeal cells (hLMCs) and studied the effects on gene expression, proliferation, cytokine production, and chemoresistance.
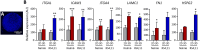
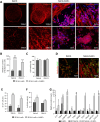
Results: In xenografted mice, ALL cells infiltrated the CNS at an early stage and, after crossing an ER-TR7+ fibroblast-like meningeal cell layer, they proliferated extensively and formed large vascularised leukaemic aggregates supported by a network of podoplanin+ leptomeningeal cells. In leukaemia spheroid-hLMC co-cultures, unlike conventional 2D co-cultures, meningeal cells strongly promoted the proliferation of leukaemic cells and generated a pro-inflammatory microenvironment. Furthermore, in 3D cell aggregates, leukaemic cells also developed chemoresistance, at least in part due to ABC transporter up-regulation.
Conclusion: Our results provide evidence for the formation of metastatic ALL-leptomeningeal cell aggregates, their pro-inflammatory profile and their contribution to leukaemic cell expansion, survival and chemoresistance in the CNS.
Keywords: 3D cell spheroids; Acute lymphoblastic leukaemia; Cell expansion; Central nervous system; Chemoresistance; Meningeal cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All animal experimentation was conducted in accordance with the Spanish guidelines for care and use of laboratory animals and protocols approved by the Complutense University and Community of Madrid (PROEX 015/19; PROEX 204.2/22). BCP-ALL samples were provided by the Onco-Haematology Unit at Niño Jesús University Children’s Hospital. Informed consent was provided according to the Declaration of Helsinki, and the study was approved by the Ethics Committee of Clinical Research at Niño Jesús Hospital (R-0009/22). Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
The choroid plexus stroma constitutes a sanctuary for paediatric B-cell precursor acute lymphoblastic leukaemia in the central nervous system.J Pathol. 2020 Oct;252(2):189-200. doi: 10.1002/path.5510. Epub 2020 Aug 28. J Pathol. 2020. PMID: 32686161 Free PMC article.
-
CXCR4 antagonists disrupt leukaemia-meningeal cell adhesion and attenuate chemoresistance.Br J Haematol. 2023 May;201(3):459-469. doi: 10.1111/bjh.18607. Epub 2022 Dec 19. Br J Haematol. 2023. PMID: 36535585 Free PMC article.
-
CD99 antibody disrupts T-cell acute lymphoblastic leukemia adhesion to meningeal cells and attenuates chemoresistance.Sci Rep. 2021 Dec 21;11(1):24374. doi: 10.1038/s41598-021-03929-x. Sci Rep. 2021. PMID: 34934147 Free PMC article.
-
A review of leptomeningeal metastases in pediatrics.J Child Neurol. 1995 May;10(3):191-9. doi: 10.1177/088307389501000304. J Child Neurol. 1995. PMID: 7642887 Review.
-
Molecular mechanisms involved in chemoresistance in paediatric acute lymphoblastic leukaemia.Srp Arh Celok Lek. 2008 Mar-Apr;136(3-4):187-92. doi: 10.2298/sarh0804187s. Srp Arh Celok Lek. 2008. PMID: 18720757 Review.
References
-
- M.T. Williams, Y.M. Yousafzai, A. Elder, K. Rehe, S. Bomken, L. Frishman-Levy, S. Tavor, P. Sinclair, K. Dormon, D. Masic, T. Perry, V.J. Weston, P. Kearns, H. Blair, L.J. Russell, O. Heidenreich, J.A. Irving, S. Izraeli, J. Vormoor, G.J. Graham, C. Halsey, The ability to cross the blood-cerebrospinal fluid barrier is a generic property of acute lymphoblastic leukemia blasts. Blood. 127(16), 1998–2006 (2016). 10.1182/blood-2015-08-665034 - PubMed
-
- Y.T. Cheung, N.D. Sabin, W.E. Reddick, D. Bhojwani, W. Liu, T.M. Brinkman, J.O. Glass, S.N. Hwang, D. Srivastava, C.H. Pui, L.L. Robison, M.M. Hudson, K.R. Krull, Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 3(10), e456–e466 (2016). 10.1016/S2352-3026(16)30110-7 - PMC - PubMed
-
- N.S. Phillips, K.L. Stratton, A.M. Williams, T. Ahles, K.K. Ness, H.J. Cohen, K. Edelstein, Y. Yasui, K. Oeffinger, E.J. Chow, R.M. Howell, L.L. Robison, G.T. Armstrong, W.M. Leisenring, K.R. Krull, Late-onset cognitive impairment and modifiable risk factors in adult Childhood Cancer survivors. JAMA Netw. Open. 6(5), e2316077 (2023). 10.1001/jamanetworkopen.2023.16077 - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

