Differentiation of human hyalocytes from induced pluripotent stem cells through ascorbic acid treatment
- PMID: 39937308
- PMCID: PMC11821750
- DOI: 10.1007/s13577-025-01182-2
Differentiation of human hyalocytes from induced pluripotent stem cells through ascorbic acid treatment
Abstract
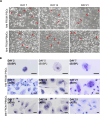
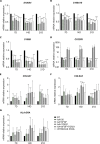
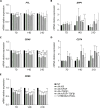
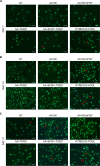
Hyalocytes are macrophage-like cells residing in the eye vitreous cortex. Even though hyalocytes have been firstly described in the mid-Nineteenth century, they have been poorly explored. Recent researches highlighted hyalocyte involvement in both physiological and pathological processes of the vitreoretinal interface. Nonetheless, the majority of works involving hyalocyte cultures were carried out in animals, while fewer studies were performed on humans because their isolation requires vitrectomy. The aim of this study was to differentiate human induced pluripotent stem cells (iPSCs) into hyalocytes as a non-invasive method to continuously obtain cells. iPSCs were first differentiated into hematopoietic stem/progenitor cells (HSPCs) and then into macrophages. Macrophages were either left untreated (NT) or treated with ascorbic acid (AA) alone or combined with bFGF and/or TGF-β1. Additionally, macrophages were cultured in the presence of a pool of vitreous bodies from vitrectomies. Cells were analyzed for morphology and then for gene and protein expression through qRT-PCR, immunofluorescence, Western Blot, and flow cytometry. Similar to cells treated with the vitreous body, macrophages treated with AA alone or in combination with bFGF exhibited a more elongated shape compared to NT or cells treated with TGF-β1. Additionally, these treatments resulted in gene expression downregulation for S100A4, S100A10, S100B, and CX3CR1, while upregulating COL6A1, HLA-DRA, and CD74. At the protein level, S100B, CD14, and CD49d were downregulated with all treatments, while collagen VI and HLA-DR were upregulated. This work demonstrates that hyalocytes can be differentiated by treatment of iPSC-derived macrophages with ascorbic acid for a period of 21 days.
Keywords: Ascorbic acid; Hyalocytes; Induced pluripotent stem cells; Macrophages; Vitreous body.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no financial or non-financial interests to disclose. Ethics approval and consent to participate: The PaViCoRe study was approved by the ethics committee of the ASST Spedali Civili di Brescia in agreement with Helsinki declaration (IRB n. NP 4365). Written informed consent was obtained from the participants. Consent to publish: Not applicable.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

