Heart-on-a-Miniscope: A Miniaturized Solution for Electrophysiological Drug Screening in Cardiac Organoids
- PMID: 39937454
- PMCID: PMC11817906
- DOI: 10.1002/smll.202409571
Heart-on-a-Miniscope: A Miniaturized Solution for Electrophysiological Drug Screening in Cardiac Organoids
Abstract
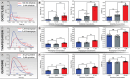
Cardiovascular toxicity remains a primary concern in drug development, accounting for a significant portion of post-market drug withdrawals due to adverse reactions such as arrhythmias. Traditional preclinical models, predominantly based on animal cells, often fail to replicate human cardiac physiology accurately, complicating the prediction of drug-induced effects. Although human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) provide a more genetically relevant system, their use in 2D, static cultures does not sufficiently mimic the dynamic, 3D environment of the human heart. 3D cardiac organoids made from human iPSC-CMs can potentially bridge this gap. However, most traditional electrophysiology assays, developed for single cells or 2D monolayers, are not readily adaptable to 3D organoids. This study uses optical calcium analysis of human organoids combined with miniaturized fluorescence microscopy (miniscope) and heart-on-a-chip technology. This simple, inexpensive, and efficient platform provides robust on-chip calcium imaging of human cardiac organoids. The versatility of the system is demonstrated through cardiotoxicity assay of drugs known to impact cardiac electrophysiology, including dofetilide, quinidine, and thapsigargin. The platform promises to advance drug testing by providing a more reliable and physiologically relevant assessment of cardiovascular toxicity, potentially reducing drug-related adverse effects in clinical settings.
Keywords: calcium imaging; cardiac organoids; cardiotoxicity; miniaturized fluorescence imaging; miniscope.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

