Once-Weekly Semaglutide in Adults With Alcohol Use Disorder: A Randomized Clinical Trial
- PMID: 39937469
- PMCID: PMC11822619
- DOI: 10.1001/jamapsychiatry.2024.4789
Once-Weekly Semaglutide in Adults With Alcohol Use Disorder: A Randomized Clinical Trial
Abstract
Importance: Preclinical, observational, and pharmacoepidemiology evidence indicates that glucagon-like peptide 1 receptor agonists (GLP-1RAs) may reduce alcohol intake. Randomized trials are needed to determine the clinical significance of these findings.
Objective: To evaluate the effects of once-weekly subcutaneous semaglutide on alcohol consumption and craving in adults with alcohol use disorder (AUD).
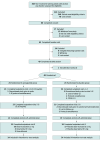
Design, setting, and participants: This was a phase 2, double-blind, randomized, parallel-arm trial involving 9 weeks of outpatient treatment. Enrollment occurred at an academic medical center in the US from September 2022 to February 2024. Of 504 potential participants assessed, 48 non-treatment-seeking participants with AUD were randomized.
Intervention: Participants received semaglutide (0.25 mg/week for 4 weeks, 0.5 mg/week for 4 weeks, and 1.0 mg for 1 week) or placebo at weekly clinic visits.
Main outcomes and measures: The primary outcome was laboratory alcohol self-administration, measured at pretreatment and posttreatment (0.5 mg/week). Secondary and exploratory outcomes, including prospective changes in alcohol consumption and craving, were assessed at outpatient visits.
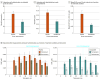
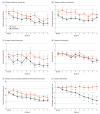
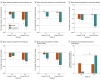
Results: Forty-eight participants (34 [71%] female; mean [SD] age, 39.9 [10.6] years) were randomized. Low-dose semaglutide reduced the amount of alcohol consumed during a posttreatment laboratory self-administration task, with evidence of medium to large effect sizes for grams of alcohol consumed (β, -0.48; 95% CI, -0.85 to -0.11; P = .01) and peak breath alcohol concentration (β, -0.46; 95% CI, -0.87 to -0.06; P = .03). Semaglutide treatment did not affect average drinks per calendar day or number of drinking days, but significantly reduced drinks per drinking day (β, -0.41; 95% CI, -0.73 to -0.09; P = .04) and weekly alcohol craving (β, -0.39; 95% CI, -0.73 to -0.06; P = .01), also predicting greater reductions in heavy drinking over time relative to placebo (β, 0.84; 95% CI, 0.71 to 0.99; P = .04). A significant treatment-by-time interaction indicated that semaglutide treatment predicted greater relative reductions in cigarettes per day in a subsample of individuals with current cigarette use (β, -0.10; 95% CI, -0.16 to -0.03; P = .005).
Conclusions and relevance: These findings provide initial prospective evidence that low-dose semaglutide can reduce craving and some drinking outcomes, justifying larger clinical trials to evaluate GLP-1RAs for alcohol use disorder.
Trial registration: ClinicalTrials.gov Identifier: NCT05520775.
Conflict of interest statement
Figures
References
-
- Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders. World Health Organization; 2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

