Anti-Inflammatory Macrophage-Derived Exosomes Modified With Self-Antigen Peptides for Treatment of Experimental Autoimmune Encephalomyelitis
- PMID: 39937659
- PMCID: PMC11967809
- DOI: 10.1002/advs.202415265
Anti-Inflammatory Macrophage-Derived Exosomes Modified With Self-Antigen Peptides for Treatment of Experimental Autoimmune Encephalomyelitis
Abstract
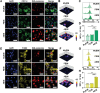
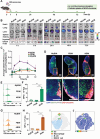
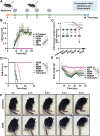
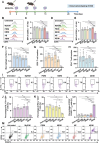
Current treatments for autoimmune diseases often involve broad-acting immunosuppressants, which carry risks such as infections and malignancies. This study investigates whether exosomes derived from anti-inflammatory macrophages (AE) and decorated with myelin oligodendrocyte glycoprotein (MOG) peptide (AE/M) can induce immune tolerance in autoimmune diseases. Experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis, serves as the autoimmune disease model. Exosomes derived from myoblasts or fibroblasts are also modified with MOG peptides for comparison. Unlike their myoblast or fibroblast counterparts, exosomes from anti-inflammatory macrophages demonstrate a targeted capacity toward antigen-presenting cells. Moreover, AE/M uniquely promotes the differentiation of dendritic cells (DC) into a tolerogenic phenotype. When splenocytes are treated with AE/M, an increased population of tolerogenic DC (tolDC) is observed, even under proinflammatory stimuli. Subcutaneous administration of AE/M in the EAE mouse model results in MOG peptide-specific immune tolerance and preserves motor coordination. In contrast to treatments with fibroblast- or myoblast-derived exosomes modified with MOG peptides, AE/M treatment provides complete protection from EAE in mice. These findings highlight the potential of self-antigen modified AE as a versatile and adaptable nanoplatform for the treatment of various autoimmune diseases.
Keywords: anti‐inflammatory macrophage‐derived exosome; autoimmune disease; encephalomyelitis; immune tolerance; self‐antigen modification.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Stauffer W. M., Alpern J. D., Walker P. F., JAMA, J. Am. Med. Assoc. 2020, 324, 623. - PubMed
MeSH terms
Substances
Grants and funding
- KEIT 20018560/Alchemist Project of the Korea Evaluation Institute of Industrial Technology (KEIT), Ministry of Trade, Industry and Energy, Republic of Korea
- NTIS 2410005252/Alchemist Project of the Korea Evaluation Institute of Industrial Technology (KEIT), Ministry of Trade, Industry and Energy, Republic of Korea
- NRF-2018R1A5A2024425/National Research Foundation (NRF), Ministry of Science and ICT, Republic of Korea
- RS-2024-00350161/National Research Foundation (NRF), Ministry of Science and ICT, Republic of Korea
LinkOut - more resources
Full Text Sources
