Structural characterization of influenza group 1 chimeric hemagglutinins as broad vaccine immunogens
- PMID: 39937865
- PMCID: PMC11848309
- DOI: 10.1073/pnas.2416628122
Structural characterization of influenza group 1 chimeric hemagglutinins as broad vaccine immunogens
Abstract
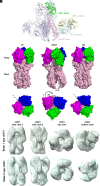
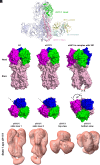
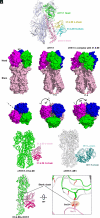
Chimeric hemagglutinins (cHA) appear to be promising for the design and development of universal influenza vaccines. Influenza A group 1 cHAs, cH5/1, cH8/1, and cH11/1, comprising an H1 stem attached to either an H5, H8, or H11 globular head, have been used sequentially as vaccine immunogens in human clinical trials and induced high levels of broadly protective antibodies. Using X-ray crystallography and negative-stain electron microscopy, we determined structures of cH5/1, cH8/1, and cH11/1 HAs in their apo (unliganded) and antibody Fab-bound states. Stem-reactive antibodies 3E1 and 31.b.09 recognize their cognate epitopes in cH5/1, cH8/1, and cH11/1 HAs. However, with cH5/1, the head domains are rotated by 35 to 45° around the threefold axis of the HA trimer compared to native HA with a more splayed-open conformation at the stem base. cH11/1 with 3E1 is structurally more native-like but resembles cH5/1 with 31.b.09, whereas cH8/1 with 31.b.09 exhibited a range of closed-to-open stem configurations with some separation of head and stem domains. Furthermore, all of these group 1 cHAs effectively bound a broad head trimer interface antibody and other broad stem antibodies. Thus, the cHAs exhibit structural plasticity without compromising the stem and head trimer interface epitopes for elicitation of influenza A group 1 cross-reactive antibodies.
Keywords: HA trimer interface and stem; X-ray crystallography; chimeric group 1 influenza hemagglutinin; negative-stain electron microscopy; universal vaccine design.
Conflict of interest statement
Competing interests statement:W.S. and F.K. are co-founders and scientific advisory board members of CastleVax. F.K. has consulted for Merck, Curevac, Seqirus, GlaxoSmithKline (GSK) and Pfizer and is currently consulting for 3rd Rock Ventures, Gritstone and Avimex. The Krammer laboratory is collaborating with Dynavax on influenza vaccine development. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, CastleVax, to develop SARS-CoV-2 vaccines. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to influenza virus vaccines, which list P.P. and F.K. as co-inventors. The Icahn School of Medicine at Mount Sinai has also filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines, and influenza virus therapeutics which list F.K. as co-inventor. W.S. is listed as co-inventor of NDV-based SARS-CoV-2 vaccines.
Figures


References
MeSH terms
Substances
Grants and funding
- 75N91019C00051/CA/NCI NIH HHS/United States
- 75N93109C00051/HHS | NIH | NIAID | Division of Intramural Research (DIR, NIAID)
- 75N93109C00051/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P41 RR001209/RR/NCRR NIH HHS/United States
- 75N93019C00051/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

