Design of Zn-Binding Peptide(s) from Protein Fragments
- PMID: 39937972
- PMCID: PMC12002108
- DOI: 10.1002/cbic.202401014
Design of Zn-Binding Peptide(s) from Protein Fragments
Abstract
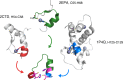
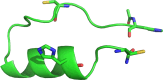
We designed a minimalistic zinc(II)-binding peptide featuring the Cys2His2 zinc-finger motif. To this aim, several tens of thousands of (His/Cys)-Xn-(His/Cys) protein fragments (n=2-20) were first extracted from the 3D protein structures deposited in Protein Data Bank (PDB). Based on geometrical constraints positioning two Cys (C) and two His (H) side chains at the vertices of a tetrahedron, approximately 22 000 sequences of the (H/C)-Xi-(H/C)-Xj-(H/C)-Xk-(H/C) type, satisfying Nmetal-binding H=Nmetal-binding C=2, were processed. Several other criteria, such as the secondary structure content and predicted fold stability, were then used to select the best candidates. To prove the viability of the computational design experimentally, three peptides were synthesized and subjected to isothermal calorimetry (ITC) measurements to determine the binding constants with Zn2+, including the entropy and enthalpy terms. For the strongest Zn2+ ions binding peptide, P1, the dissociation constant was shown to be in the nanomolar range (KD=~220 nM; corresponding to ΔGbind=-9.1 kcal mol-1). In addition, ITC showed that the [P1 : Zn2+] complex forms in 1 : 1 stoichiometry and two protons are released upon binding, which suggests that the zinc coordination involves both cysteines. NMR experiments also indicated that the structure of the [P1 : Zn2+] complex might be quite similar to the computationally predicted one. In summary, our proof-of-principle study highlights the usefulness of our computational protocol for designing novel metal-binding peptides.
Keywords: Computer design; Isothermal calorimetry; Metal-binding peptide; NMR; QM modeling; Zinc(II).
© 2025 The Author(s). ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




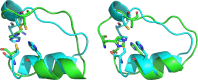

References
-
- Sánchez-Aparicio J.-E., Tiessler-Sala L., Velasco-Carneros L., Roldán-Martín L., Sciortino G., Maréchal J.-D., J. Chem. Inf. Model. 2021, 61 (1), 311–323. - PubMed
-
- Bertini I., Gray H. B., Stiefel E. I., Valentine J. S., Biological Inorganic Chemistry. Structure and Reactivity, University Science Books, 2007, ISBN 978-1-891389-43-6.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

