Exploring the Role of Macrophages and Their Associated Structures in Rheumatoid Arthritis
- PMID: 39938504
- PMCID: PMC11820663
- DOI: 10.1159/000543444
Exploring the Role of Macrophages and Their Associated Structures in Rheumatoid Arthritis
Abstract
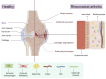
Background: Rheumatoid arthritis (RA) is a chronic, invasive autoimmune disease characterized by symmetrical polyarthritis involving synovial inflammation. Epidemiological studies indicate that the incidence of RA continues to rise, yet the pathogenesis of this disease remains not fully understood. A significant infiltration of macrophages is observed in the synovium of RA patients. It can be inferred that macrophages likely play a crucial role in the onset and progression of RA.
Summary: This review aims to summarize the research progress on the mechanisms by which macrophages and their associated structures contribute to RA, as well as potential therapeutic approaches, aiming to provide new insights into the study of RA pathogenesis and its clinical treatment.
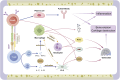
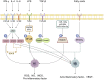
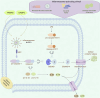
Key messages: During the course of RA, besides the inherent roles of macrophages, these cells respond to microenvironmental changes such as pathogen invasion or tissue damage by undergoing polarization, pyroptosis, or forming macrophage extracellular traps (METs), all of which influence inflammatory responses and immune homeostasis, thereby mediating the occurrence and development of RA. Additionally, macrophages secrete exosomes, which participate in intercellular communication and signal transduction processes, thus contributing to the progression of RA. Therefore, it is critical to elucidate how macrophages and their related structures function in RA.
Background: Rheumatoid arthritis (RA) is a chronic, invasive autoimmune disease characterized by symmetrical polyarthritis involving synovial inflammation. Epidemiological studies indicate that the incidence of RA continues to rise, yet the pathogenesis of this disease remains not fully understood. A significant infiltration of macrophages is observed in the synovium of RA patients. It can be inferred that macrophages likely play a crucial role in the onset and progression of RA.
Summary: This review aims to summarize the research progress on the mechanisms by which macrophages and their associated structures contribute to RA, as well as potential therapeutic approaches, aiming to provide new insights into the study of RA pathogenesis and its clinical treatment.
Key messages: During the course of RA, besides the inherent roles of macrophages, these cells respond to microenvironmental changes such as pathogen invasion or tissue damage by undergoing polarization, pyroptosis, or forming macrophage extracellular traps (METs), all of which influence inflammatory responses and immune homeostasis, thereby mediating the occurrence and development of RA. Additionally, macrophages secrete exosomes, which participate in intercellular communication and signal transduction processes, thus contributing to the progression of RA. Therefore, it is critical to elucidate how macrophages and their related structures function in RA.
Keywords: Macrophage exosome; Macrophage extracellular traps; Macrophage polarization; Macrophage pyroptosis; Rheumatoid arthritis.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
DS-Modified Paeoniflorin pH-Responsive Lipid-Polymer Hybrid Nanoparticles for Targeted Macrophage Polarization in a Rat Model of Rheumatoid Arthritis.Int J Nanomedicine. 2025 Jul 12;20:8967-8992. doi: 10.2147/IJN.S516434. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40671689 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Low-Protein Diet Inhibits the Synovial Tissue Macrophage Pro-Inflammatory Polarization Via NRF2/SIRT3/SOD2/ROS Pathway in K/BxN Rheumatoid Arthritis Mice.Inflammation. 2025 Aug;48(4):1689-1703. doi: 10.1007/s10753-024-02145-9. Epub 2024 Sep 18. Inflammation. 2025. PMID: 39292325
-
The immunomodulatory of interleukin-33 in rheumatoid arthritis: A systematic review.Clin Immunol. 2024 Aug;265:110264. doi: 10.1016/j.clim.2024.110264. Epub 2024 May 31. Clin Immunol. 2024. PMID: 38825072
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Venetsanopoulou AI, Alamanos Y, Voulgari PV, Drosos AA. Epidemiology of rheumatoid arthritis: genetic and environmental influences. Expert Rev Clin Immunol. 2022;18(9):923–31. - PubMed
-
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. . Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40. - PubMed
-
- Tardito S, Martinelli G, Soldano S, Paolino S, Pacini G, Patane M, et al. . Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18(11):102397. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

