Controlling the local extracellular electric field can suppress the generation and propagation of seizures and spikes in the hippocampus
- PMID: 39938862
- PMCID: PMC12013223
- DOI: 10.1016/j.brs.2025.02.001
Controlling the local extracellular electric field can suppress the generation and propagation of seizures and spikes in the hippocampus
Abstract
Objective: Neural activity such as theta waves, epileptic spikes and seizures can cross a physical transection using electric fields thus propagating by ephaptic coupling and independently of synaptic transmission. Recruitment of neurons in epilepsy occurs in part due to electric field coupling in addition to synaptic mechanisms. Hence, controlling the local electric field could suppress or cancel the generation of these epileptic events.
Methods: 4-aminopyridine (4-AP) was used to induce spontaneous epileptic spikes and seizures in longitudinal hippocampal slices in-vitro. Two extracellular recording electrodes were placed in the tissue, one at the edge of the slice on the temporal side at the focus of the epileptic activity and the other on the septal side to record the propagation. Two stimulating electrodes were placed outside the slice at the edge of the focal zone. An extracellular voltage clamp circuit maintained the voltage within the focus at 0V with respect to the bath ground.
Results: Experiments showed that 100 % of the epileptic activity originated at the temporal region and propagated to the septal region of the slices thereby establishing the existence of a focus in the temporal end of the tissue. The clamp achieved 100 % suppression of all seizure activity in the tissue with current amplitudes between 70 and 250 nA. No spikes or seizures were observed in either the focus or the septal region when the clamp was "on". When the clamp was turned off, both the spikes and seizure events recovered immediately.
Conclusions: The experiments show that controlling the extracellular voltage within a focus can prevent the generation and the propagation of epileptiform activity from the focus with very low amplitudes currents.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures


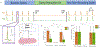



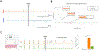
Similar articles
-
Theta waves, neural spikes and seizures can propagate by ephaptic coupling in vivo.Exp Neurol. 2022 Aug;354:114109. doi: 10.1016/j.expneurol.2022.114109. Epub 2022 May 10. Exp Neurol. 2022. PMID: 35551899 Free PMC article.
-
Neural recruitment by ephaptic coupling in epilepsy.Epilepsia. 2021 Jul;62(7):1505-1517. doi: 10.1111/epi.16903. Epub 2021 May 12. Epilepsia. 2021. PMID: 33979453
-
Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields.Exp Neurol. 2019 Jul;317:119-128. doi: 10.1016/j.expneurol.2019.02.005. Epub 2019 Feb 15. Exp Neurol. 2019. PMID: 30776338
-
Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge.Epilepsia. 2001;42 Suppl 3:2-4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. Epilepsia. 2001. PMID: 11520313 Review.
-
Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro.Prog Neurobiol. 2002 Oct;68(3):167-207. doi: 10.1016/s0301-0082(02)00077-1. Prog Neurobiol. 2002. PMID: 12450487 Review.
References
-
- Gloor P, Vera CL, Sperti L. Electrophysiological studies of hippocampal neurons I. Configuration and laminar analysis of the “resting” potential gradient, of the main-transient response to perforant path, fimbrial and mossy fiber volleys and of “spontaneous” activity. Electroencephalogr Clin Neurophysiol 1963;15:353–78. - PubMed
-
- Green JD, Petsche H. Hippocampal electrical activity IV. Abnormal electrical activity. Electroencephalogr Clin Neurophysiol 1961;13:868–79.
-
- Jefferys JGR. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev 1995;75:689–723. - PubMed
-
- Swann JW, Brady RJ, Friedman RJ, Smith EJ. The dendritic origins of penicillin-induced epileptogenesis in CA3 hippocampal pyramidal cells. J Neurophysiol 1986;56:1718–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

