Implementing Guidelines for hypothermia prevention with Local adaptation to keep periOperative patients Warm (GLOW): protocol of a stepped-wedge cluster randomised hybrid type II effectiveness-implementation study
- PMID: 39938961
- PMCID: PMC11822429
- DOI: 10.1136/bmjopen-2024-091577
Implementing Guidelines for hypothermia prevention with Local adaptation to keep periOperative patients Warm (GLOW): protocol of a stepped-wedge cluster randomised hybrid type II effectiveness-implementation study
Abstract
Introduction: Perioperative hypothermia is a common and preventable complication of surgery. Systems level change that enables perioperative teams to integrate hypothermia prevention into practice in ways that are contextually appropriate is needed. The purpose of this trial is to evaluate the effectiveness and implementation of perioperative hypothermia prevention guidance with local adaptation on clinical, implementation and economic outcomes. Our objective is to decrease the risk of patients developing perioperative hypothermia.
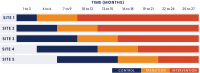
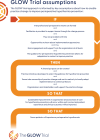
Methods and analysis: A hybrid type II effectiveness-implementation study. An incomplete stepped-wedge cluster randomised controlled trial design will be used, with a 6-month transition period for implementation. Perioperative departments from five major public hospitals in South East Queensland, Australia, will participate over 27 months. The co-primary outcomes are (effectiveness) hypothermia on arrival to the post anaesthetic care unit (PACU) and (implementation) extent of temperature monitoring and active warming. Secondary clinical effectiveness outcomes include hypothermia at any perioperative time point, PACU and hospital length of stay, intraoperative or post-anaesthetic adverse events, blood transfusions and surgical site infection. Secondary implementation outcomes include pre-transition measures of adoptability and implementability, and post-transition measures of adoption, fidelity of implementation strategy and site team learning. Cost-effectiveness will evaluate implementation costs and quality-adjusted life years. Based on the number and unequal sizes of clusters, we used a constrained randomisation approach for sample size determination to minimise the imbalance between control and intervention period sample sizes. Generalised linear mixed models will be used to analyse primary and secondary outcomes.
Ethics and dissemination: Ethical approval was obtained with a waiver of consent to access clinical records (reference: HREC/2023/MNHB/94571). Informed consent will be sought from patients completing surveys. Consent will be implied from clinicians participating in implementation evaluation. Findings will be disseminated through journal publications and conference presentations. Practice and policy recommendations will be collaboratively developed with partner organisations.
Trial registration number: Prospective registration of the trial was obtained on 28 July 2023 on the Australia and New Zealand Clinical Trials Registry Network: ACTRN12623000814673.
Keywords: ANAESTHETICS; Clinical Trial; Implementation Science; Nursing Care; SURGERY.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: JM, JD, DS and NR have received honorariums from 3M for educational consultancies. JM, and JD’s employers have received grants from 3M for the development of the perioperative hypothermia prevention guidance which will be implemented in this study. All other authors have no competing interest to declare.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical