The dual role of VEGF-A in a complex in vitro model of oxaliplatin-induced neurotoxicity: Pain-related and neuroprotective effects
- PMID: 39939241
- PMCID: PMC12014407
- DOI: 10.1016/j.neurot.2025.e00532
The dual role of VEGF-A in a complex in vitro model of oxaliplatin-induced neurotoxicity: Pain-related and neuroprotective effects
Abstract
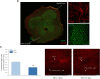
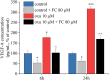
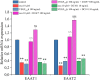
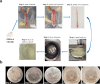
Vascular endothelial growth factor (VEGF)-A is a main player in the development of neuropathic pain induced by chemotherapy and the pharmacological blockade of VEGF receptor (VEGFR) subtype 1 is a pain killer strategy. Interestingly, VEGF-A has been demonstrated to have also neuroprotective properties. The aim of the study was to investigate the neuroprotective role of VEGF-A against oxaliplatin neurotoxicity, attempting to discriminate pain-related and restorative signaling pathways. We used rat organotypic spinal cord slices treated with oxaliplatin, as an in vitro model to study chemotherapy-induced toxicity. In this model, 10 μM oxaliplatin caused a time-dependent release of VEGF-A, which was reduced by the astrocyte inhibitor fluorocitrate. Moreover, glia inhibition exacerbated oxaliplatin-induced cytotoxicity in a VEGF-A sensitive manner. Treatment with VEGF165b, the main isoform of VEGF-A, prevented the oxaliplatin-induced neuronal damage (indicated by NeuN staining) and astrocyte activation (indicated by GFAP staining). In addition, the blockade of VEGFR-2 by the selective antibody DC101 blunted the protective action of VEGF165b. In the same model, VEGF165b increased the release of molecules relevant in pain signaling, like substance P and CGRP, as well as the mRNA expression of glutamate transporters (EAAT1 and EAAT2), similarly to oxaliplatin and these effects were prevented by the selective VEGFR-1 blocker antibody D16F7. In conclusion, VEGF-A plays a dichotomic role in an in vitro model of chemotherapy-induced toxicity, either promoting neuroprotection or triggering pain mediators release, depending on which of its two receptors is activated. The selective management of VEGF-A signaling is suggested as a therapeutic approach.
Keywords: Chemotherapy-induced neurotoxicity; Glial cells; Neuroprotection; Spinal cord organotypic slices; VEGF-A; VEGFRs.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures









References
-
- Seretny M., Currie G.L., Sena E.S., Ramnarine S., Grant R., MacLeod M.R., et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014 Dec;155(12):2461–2470. - PubMed
-
- Aldskogius H., Kozlova E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998 May;55(1):1–26. - PubMed
-
- Volterra A., Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005 Aug;6(8):626–640. - PubMed
-
- Haydon P.G., Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–1031. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

