Cytisine for smoking cessation in hospitalised smokers with cardiovascular diseases: an observational study
- PMID: 39939444
- PMCID: PMC12009246
- DOI: 10.1007/s11739-025-03888-5
Cytisine for smoking cessation in hospitalised smokers with cardiovascular diseases: an observational study
Erratum in
-
Correction: Cytisine for smoking cessation in hospitalised smokers with cardiovascular diseases: an observational study.Intern Emerg Med. 2025 Sep;20(6):1987. doi: 10.1007/s11739-025-04067-2. Intern Emerg Med. 2025. PMID: 40826192 Free PMC article. No abstract available.
Abstract
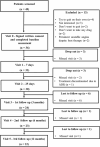
Cigarette smoke is a significant risk factor for cardiovascular diseases (CVD). Among pharmacotherapy for smoking cessation, the plant alkaloid cytisine (CYT) -a nicotinic receptors partial agonist- has been shown to have a safe profile, with a minimal risk for drug interactions. Since previous studies have excluded CVD patients, there are few existing data examining CYT safety in this critical population. An observational prospective study was conducted in the Verona University Hospital (AOUI), Italy, to assess the safety and efficacy of CYT for smoking cessation. Thirty-six hospitalised participants from the Cardiology Department received oral CYT 1.5 mg for 25 days, according to the West Dosing Schedule (6 capsules for the first 3 days, gradually decreased up to 2 capsules on the last 6 days), in combination with supportive care. The primary endpoint was CYT safety, with 11 mild-moderate Adverse Drug Reactions (ADRs) reported by 9 (25%) participants. Initial insomnia (11%), nausea (6%), sleep disorders (6%), headache (3%), gastritis (3%), and diarrhoea (3%) were the most frequent symptoms. No serious or unexpected ADRs were identified, with no increase in cardiovascular events. Efficacy was assessed as self-reported 7-day point prevalence abstinence (PPA) at 3, 6 and 12 months post-quit. At the first follow-up, abstinence was also biochemically verified by exhaled carbon monoxide (CO) measurement, which was confirmed for 36% of participants. Considering lost to follow-up as relapsed patients, the PPA was 50%, 47% and 36% at 1st, 2nd and 3rd follow-up, respectively. These results may suggest that CYT has a well-established safety profile in hospitalised CVD patients, but further investigation is needed.
Keywords: Addiction; Cardiovascular disease; Cytisine; Hospital; Safety; Smoking cessation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest. Ethical approval: This study protocol was approved by the Local Ethics Committee on September 23rd 2021 (reference protocol number 55360-3439CESC). This study was carried out in accordance with the principles expressed in the Declaration of Helsinki, and we ensured protection of patient privacy and anonymity. Informed consent: Written informed consent was obtained from patients or their surrogates before enrollment.
Figures

References
-
- “Cardiovascular Diseases - The Health Consequences of Smoking—50 Years of Progress - NCBI Bookshelf.” Accessed: May 06, 2024. [Online]. https://www.ncbi.nlm.nih.gov/books/NBK294323/
-
- Morris PB et al (2015) Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the american college of cardiology. J Am Coll Cardiol 66(12):1378–1391. 10.1016/J.JACC.2015.07.037 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

