PPP2R1A mutations cause ATR inhibitor sensitivity in ovarian clear cell carcinoma
- PMID: 39939726
- PMCID: PMC11850283
- DOI: 10.1038/s41388-024-03265-0
PPP2R1A mutations cause ATR inhibitor sensitivity in ovarian clear cell carcinoma
Abstract
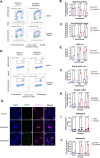
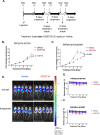
Identification of ARID1A/ATR synthetic lethality led to ATR inhibitor phase II trials in ovarian clear cell carcinoma (OCCC), a cancer of unmet need. Using multiple CRISPR-Cas9 mutagenesis and interference screens, we show that inactivation of protein phosphatase 2A (PP2A) subunits, including PPP2R1A, enhance ATRi sensitivity in ARID1A mutant OCCC. Analysis of a new OCCC cohort indicates that 52% possess oncogenic PPP2R1A p.R183 mutations and of these, one half possessed both ARID1A as well as PPP2R1A mutations. Using CRISPR-prime editing to generate new isogenic models of PPP2R1A mutant OCCC, we found that PPP2R1A p.R183W and p.R183P mutations cause ATRi-induced S phase stress, premature mitotic entry, genomic instability and ATRi sensitivity in OCCC tumour cells. p.R183 mutation also enhanced both in vitro and in vivo ATRi sensitivity in preclinical models of ARID1A mutant OCCC. These results argue for the assessment of PPP2R1A mutations as a biomarker of ATRi sensitivity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: CJL makes the following disclosures: receives and/or has received research funding from: AstraZeneca, Merck KGaA, Artios, Neophore. Received consultancy, SAB membership or honoraria payments from: FoRx, Syncona, Sun Pharma, Gerson Lehrman Group, Merck KGaA, Vertex, AstraZeneca, Tango Therapeutics, 3rd Rock, Ono Pharma, Artios, Abingworth, Tesselate, Dark Blue Therapeutics, Pontifax, Astex, Neophore, Glaxo Smith Kline, Dawn Bioventures, Blacksmith Medicines, FoRx Therapeutics, Ariceum. Has stock in: Tango, Ovibio, Hysplex, Tesselate. CJL is also a named inventor on patents describing the use of DNA repair inhibitors and stands to gain from their development and use as part of the ICR “Rewards to Inventors” scheme and also reports benefits from this scheme associated with patents for PARP inhibitors paid into CJL’s personal account and research accounts at the Institute of Cancer Research. SB makes the following disclosure: receives and/or has received research funding from: AstraZeneca. Received consultancy, SAB membership or honoraria payments from Abbvie, Astrazeneca, Biontech, Eisai, Gilead, GlaxoSmithKline, Grey Wolf Therapeutics, Immunogen, Incyte, ITM Oncologics, Merck Sharpe Dohme, Mersana, Myriad, Oncxerna, Pharmaand, Seagen, Takeda. Verastem, Zymeworks.
Figures


References
-
- Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–6. - PubMed
-
- McCluggage WG. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61:152–63. - PubMed
-
- Okamoto A, Glasspool RM, Mabuchi S, Matsumura N, Nomura H, Itamochi H, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014;24:S20–25. - PubMed
-
- Pather S, Quinn MA. Clear-cell cancer of the ovary-is it chemosensitive? Int J Gynecol Cancer. 2005;15:432–7. - PubMed
-
- Takano M, Sugiyama T, Yaegashi N, Sakuma M, Suzuki M, Saga Y, et al. Low response rate of second-line chemotherapy for recurrent or refractory clear cell carcinoma of the ovary: a retrospective Japan Clear Cell Carcinoma Study. Int J Gynecol Cancer. 2008;18:937–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

