UM171 glues asymmetric CRL3-HDAC1/2 assembly to degrade CoREST corepressors
- PMID: 39939761
- PMCID: PMC11882444
- DOI: 10.1038/s41586-024-08532-4
UM171 glues asymmetric CRL3-HDAC1/2 assembly to degrade CoREST corepressors
Abstract
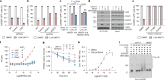
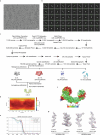
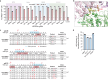
UM171 is a potent agonist of ex vivo human haematopoietic stem cell self-renewal1. By co-opting KBTBD4, a substrate receptor of the CUL3-RING E3 ubiquitin ligase (CRL3) complex, UM171 promotes the degradation of the LSD1-CoREST corepressor complex, thereby limiting haematopoietic stem cell attrition2,3. However, the direct target and mechanism of action of UM171 remain unclear. Here we show that UM171 acts as a molecular glue to induce high-affinity interactions between KBTBD4 and HDAC1/2 to promote corepressor degradation. Through proteomics and chemical inhibitor studies, we identify the principal target of UM171 as HDAC1/2. Cryo-electron microscopy analysis of dimeric KBTBD4 bound to UM171 and the LSD1-HDAC1-CoREST complex identifies an asymmetric assembly in which a single UM171 molecule enables a pair of KELCH-repeat propeller domains to recruit the HDAC1 catalytic domain. One KBTBD4 propeller partially masks the rim of the HDAC1 active site, which is exploited by UM171 to extend the E3-neosubstrate interface. The other propeller cooperatively strengthens HDAC1 binding through a distinct interface. The overall CoREST-HDAC1/2-KBTBD4 interaction is further buttressed by the endogenous cofactor inositol hexakisphosphate, which acts as a second molecular glue. The functional relevance of the quaternary complex interaction surfaces is demonstrated by base editor scanning of KBTBD4 and HDAC1. By delineating the direct target of UM171 and its mechanism of action, we reveal how the cooperativity offered by a dimeric CRL3 E3 can be leveraged by a small molecule degrader.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.B.L. is a cofounder, shareholder and member of the scientific advisory board of Light Horse Therapeutics. N.Z. is one of the scientific cofounders and a shareholder of SEED Therapeutics. N.Z. serves as a member of the scientific advisory board of Synthex, Molecular Glue Labs and Differentiated Therapeutics with financial interests. R.M. and N.C.P. are listed as inventors on patent applications related to the CoraFluor TR-FRET probes used in this work. P.A.C. is a co-founder of Acylin Therapeutics and a consultant for Abbvie regarding p300 acetyltransferase inhibitors. L.B.-P. is a founder, consultant and holds privately held equity in Scorpion Therapeutics. R.M. is a scientific advisory board member and equity holder of Regenacy Pharmaceuticals. S.A.C. is a member of the scientific advisory boards of Kymera, PTM BioLabs, Seer and PrognomIQ. The other authors declare no competing interests.
Figures












Update of
-
Asymmetric Engagement of Dimeric CRL3 KBTBD4 by the Molecular Glue UM171 Licenses Degradation of HDAC1/2 Complexes.bioRxiv [Preprint]. 2024 May 14:2024.05.14.593897. doi: 10.1101/2024.05.14.593897. bioRxiv. 2024. Update in: Nature. 2025 Mar;639(8053):232-240. doi: 10.1038/s41586-024-08532-4. PMID: 38798619 Free PMC article. Updated. Preprint.
References
-
- Chagraoui, J. et al. UM171 preserves epigenetic marks that are reduced in ex vivo culture of human HSCs via potentiation of the CLR3-KBTBD4 complex. Cell Stem Cell28, 48–62 (2021). - PubMed
-
- Cowan, A. D. & Ciulli, A. Driving E3 ligase substrate specificity for targeted protein degradation: lessons from nature and the laboratory. Annu. Rev. Biochem.91, 295–319 (2022). - PubMed
-
- Schreiber, S. L. The rise of molecular glues. Cell184, 3–9 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

