Converging mechanism of UM171 and KBTBD4 neomorphic cancer mutations
- PMID: 39939763
- PMCID: PMC11882451
- DOI: 10.1038/s41586-024-08533-3
Converging mechanism of UM171 and KBTBD4 neomorphic cancer mutations
Abstract
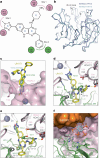
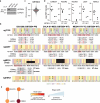
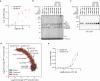
Cancer mutations can create neomorphic protein-protein interactions to drive aberrant function1,2. As a substrate receptor of the CULLIN3-RING E3 ubiquitin ligase complex, KBTBD4 is recurrently mutated in medulloblastoma3, the most common embryonal brain tumour in children4. These mutations impart gain-of-function to KBTBD4 to induce aberrant degradation of the transcriptional corepressor CoREST5. However, their mechanism remains unresolved. Here we establish that KBTBD4 mutations promote CoREST degradation through engaging HDAC1/2 as the direct target of the mutant substrate receptor. Using deep mutational scanning, we chart the mutational landscape of the KBTBD4 cancer hotspot, revealing distinct preferences by which insertions and substitutions can promote gain-of-function and the critical residues involved in the hotspot interaction. Cryo-electron microscopy analysis of two distinct KBTBD4 cancer mutants bound to LSD1-HDAC1-CoREST reveals that a KBTBD4 homodimer asymmetrically engages HDAC1 with two KELCH-repeat β-propeller domains. The interface between HDAC1 and one of the KBTBD4 β-propellers is stabilized by the medulloblastoma mutations, which insert a bulky side chain into the HDAC1 active site pocket. Our structural and mutational analyses inform how this hotspot E3-neosubstrate interface can be chemically modulated. First, we unveil a converging shape-complementarity-based mechanism between gain-of-function E3 mutations and a molecular glue degrader, UM171. Second, we demonstrate that HDAC1/2 inhibitors can block the mutant KBTBD4-HDAC1 interface and proliferation of KBTBD4-mutant medulloblastoma cells. Altogether, our work reveals the structural and mechanistic basis of cancer mutation-driven neomorphic protein-protein interactions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.B.L. is a co-founder, shareholder and member of the scientific advisory board of Light Horse Therapeutics. N.Z. is one of the scientific cofounders and a shareholder of SEED Therapeutics. N.Z. serves as a member of the scientific advisory board of Synthex, Molecular Glue Lab and Differentiated Therapeutics with financial interests. R.M. is a scientific advisory board member and equity holder of Regenacy Pharmaceuticals. R.M. and N.C.P. are inventors on patent applications related to the CoraFluor TR-FRET probes used in this work. P.A.C. is a co-founder of Acylin Therapeutics and a consultant for Abbvie regarding p300 acetyltransferase inhibitors. The remaining authors declare no competing interests.
Figures









Update of
-
KBTBD4 Cancer Hotspot Mutations Drive Neomorphic Degradation of HDAC1/2 Corepressor Complexes.bioRxiv [Preprint]. 2024 May 14:2024.05.14.593970. doi: 10.1101/2024.05.14.593970. bioRxiv. 2024. Update in: Nature. 2025 Mar;639(8053):241-249. doi: 10.1038/s41586-024-08533-3. PMID: 38798357 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

