A metagenomic 'dark matter' enzyme catalyses oxidative cellulose conversion
- PMID: 39939775
- PMCID: PMC11946906
- DOI: 10.1038/s41586-024-08553-z
A metagenomic 'dark matter' enzyme catalyses oxidative cellulose conversion
Erratum in
-
Publisher Correction: A metagenomic 'dark matter' enzyme catalyses oxidative cellulose conversion.Nature. 2025 Apr;640(8058):E7. doi: 10.1038/s41586-025-08872-9. Nature. 2025. PMID: 40119088 Free PMC article. No abstract available.
Abstract
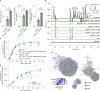
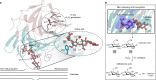
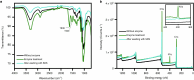
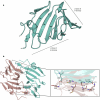
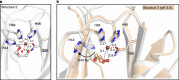
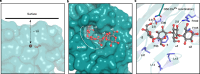
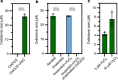
The breakdown of cellulose is one of the most important reactions in nature1,2 and is central to biomass conversion to fuels and chemicals3. However, the microfibrillar organization of cellulose and its complex interactions with other components of the plant cell wall poses a major challenge for enzymatic conversion4. Here, by mining the metagenomic 'dark matter' (unclassified DNA with unknown function) of a microbial community specialized in lignocellulose degradation, we discovered a metalloenzyme that oxidatively cleaves cellulose. This metalloenzyme acts on cellulose through an exo-type mechanism with C1 regioselectivity, resulting exclusively in cellobionic acid as a product. The crystal structure reveals a catalytic copper buried in a compact jelly-roll scaffold that features a flattened cellulose binding site. This metalloenzyme exhibits a homodimeric configuration that enables in situ hydrogen peroxide generation by one subunit while the other is productively interacting with cellulose. The secretome of an engineered strain of the fungus Trichoderma reesei expressing this metalloenzyme boosted the glucose release from pretreated lignocellulosic biomass under industrially relevant conditions, demonstrating its biotechnological potential. This discovery modifies the current understanding of bacterial redox enzymatic systems devoted to overcoming biomass recalcitrance5-7. Furthermore, it enables the conversion of agro-industrial residues into value-added bioproducts, thereby contributing to the transition to a sustainable and bio-based economy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.A.S., F.M., E.A.L., G.F.P. and M.T.M. are named inventors on patent application number BR10202401483 filed by the Brazilian Center for Research in Energy and Materials, covering the use of the enzyme discovered in this study for biomass conversion and related biotechnological applications. The other authors declare no competing interests.
Figures





References
-
- Bomble, Y. J. et al. Lignocellulose deconstruction in the biosphere. Curr. Opin. Chem. Biol.41, 61–70 (2017). - PubMed
-
- Lynd, L. R. et al. How biotech can transform biofuels. Nat. Biotechnol.26, 169–172 (2008). - PubMed
-
- Chundawat, S. P. S., Beckham, G. T., Himmel, M. E. & Dale, B. E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng.2, 121–145 (2011). - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

