Intrinsic electrical activity drives small-cell lung cancer progression
- PMID: 39939778
- PMCID: PMC11922742
- DOI: 10.1038/s41586-024-08575-7
Intrinsic electrical activity drives small-cell lung cancer progression
Abstract
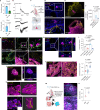
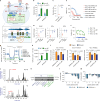
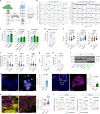
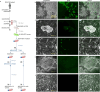
Elevated or ectopic expression of neuronal receptors promotes tumour progression in many cancer types1,2; neuroendocrine (NE) transformation of adenocarcinomas has also been associated with increased aggressiveness3. Whether the defining neuronal feature, namely electrical excitability, exists in cancer cells and impacts cancer progression remains mostly unexplored. Small-cell lung cancer (SCLC) is an archetypal example of a highly aggressive NE cancer and comprises two major distinct subpopulations: NE cells and non-NE cells4,5. Here we show that NE cells, but not non-NE cells, are excitable, and their action potential firing directly promotes SCLC malignancy. However, the resultant high ATP demand leads to an unusual dependency on oxidative phosphorylation in NE cells. This finding contrasts with the properties of most cancer cells reported in the literature, which are non-excitable and rely heavily on aerobic glycolysis. Additionally, we found that non-NE cells metabolically support NE cells, a process akin to the astrocyte-neuron metabolite shuttle6. Finally, we observed drastic changes in the innervation landscape during SCLC progression, which coincided with increased intratumoural heterogeneity and elevated neuronal features in SCLC cells, suggesting an induction of a tumour-autonomous vicious cycle, driven by cancer cell-intrinsic electrical activity, which confers long-term tumorigenic capability and metastatic potential.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Z.L. is currently an employee of Sesame Therapeutics and shareholder of Sesame Therapeutics and Tango Therapeutics. L.C. is currently an employee of AstraZeneca. B.J.D. consults for AstraZeneca, Sonata Therapeutics and Dialectic Therapeutics. J.-S.C. receives research funding from VolitionRX and AstraZeneca (Taiwan). M.G.V.H. discloses that he is a scientific advisor for Sage Therapeutics, Agios Pharmaceuticals, Auron Therapeutics, iTeos Therapeutics, Lime Therapeutics, Pretzel Therapeutics, MPM Capital and Droia Ventures. T.J. is a member of the Board of Directors of Amgen and Thermo Fisher Scientific. He is also a co-founder of Dragonfly Therapeutics and T2 Biosystems. T.J. serves on the Scientific Advisory Board of Dragonfly Therapeutics, SQZ Biotech and Skyhawk Therapeutics. None of these affiliations represent a conflict of interest with respect to the design or execution of this study or the interpretation of the data presented in this paper.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

