STING directly interacts with PAR to promote apoptosis upon acute ionizing radiation-mediated DNA damage
- PMID: 39939798
- PMCID: PMC12163073
- DOI: 10.1038/s41418-025-01457-z
STING directly interacts with PAR to promote apoptosis upon acute ionizing radiation-mediated DNA damage
Abstract
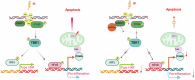
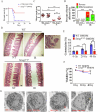
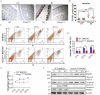
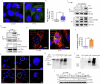
Acute ionizing radiation (IR) causes severe DNA damage, leading to cell cycle arrest, cell death, and activation of the innate immune system. The role and signaling pathway of stimulator of interferon genes (STING) in IR-induced tissue damage and cell death are not well understood. This study revealed that STING is crucial for promoting apoptosis in response to DNA damage caused by acute IR both in vitro and in vivo. STING binds to poly (ADP‒ribose) (PAR) produced by activated poly (ADP‒ribose) polymerase-1 (PARP1) upon IR. Compared with that in WT cells, apoptosis was suppressed in Stinggt-/gt- cells. Excessive PAR production by PARP1 due to DNA damage enhances STING phosphorylation, and inhibiting PARP1 reduces cell apoptosis after IR. In vivo, IR-induced crypt cell death was significantly lower in Stinggt-/gt- mice or with low-dose PARP1 inhibitor, PJ34, resulting in substantial resistance to abdominal irradiation. STING deficiency or inhibition of PARP1 function can reduce the expression of the proapoptotic gene PUMA, decrease the localization of Bax on the mitochondrial membrane, and thus reduce cell apoptosis. Our findings highlight crucial roles for STING and PAR in the IR-mediated induction of apoptosis, which may have therapeutic implications for controlling radiation-induced apoptosis or acute radiation symptoms. STING responds to acute ionizing radiation-mediated DNA damage by directly binding to poly (ADP-ribose) (PAR) produced by activated poly (ADP-ribose) polymerase-1 (PARP1), and mainly induces cell apoptosis through Puma-Bax interaction. STING deficiency or reduced production of PAR protected mice against Acute Radiation Syndrome.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev. 2022;22:124–38. - PubMed
MeSH terms
Substances
Grants and funding
- U19 AI067769/AI/NIAID NIH HHS/United States
- 2024A1515013177/Guangdong Science and Technology Department (Science and Technology Department, Guangdong Province)
- TSBICIP-KJGG-011/Natural Science Foundation of Tianjin Municipal Science and Technology Commission (Natural Science Foundation of Tianjin Municipal Science & Technology Commission)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

