Acyl-CoA thioesterase 8 induces gemcitabine resistance via regulation of lipid metabolism and antiferroptotic activity in pancreatic ductal adenocarcinoma
- PMID: 39939803
- PMCID: PMC12098905
- DOI: 10.1038/s41401-025-01477-y
Acyl-CoA thioesterase 8 induces gemcitabine resistance via regulation of lipid metabolism and antiferroptotic activity in pancreatic ductal adenocarcinoma
Abstract
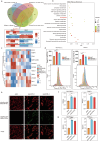
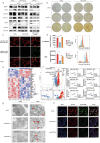
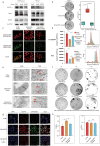
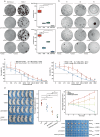
Pancreatic ductal adenocarcinoma (PDAC) comprises a group of highly malignant tumors of the pancreas. Metabolic reprogramming in tumors plays a pivotal role in promoting cancer progression. However, little is known about the metabolic alterations in tumors that drive cancer drug resistance in patients with PDAC. Here, we identified acyl-CoA thioesterase 8 (ACOT8) as a key player in driving PDAC gemcitabine (GEM) resistance. The expression of ACOT8 is significantly upregulated in GEM-resistant PDAC tissues and is closely associated with poor survival in patients with PDAC. Gain- and loss-of-function studies have shown that ACOT8 drives PDAC GEM resistance both in vitro and in vivo. Mechanistically, ACOT8 regulates cellular cholesterol ester (CE) levels, decreases the levels of phosphatidylethanolamines (PEs) that bind to polyunsaturated fatty acids and promote peroxisome activation. The knockdown of ACOT8 promotes ferroptosis and increases the chemosensitivity of tumors to GEM by inducing ferroptosis-associated pathway activation in PDAC cell lines. The combination of orlistat, an ACOT8 inhibitor, and GEM significantly inhibited tumor growth in PDAC organoid and mouse models. This study reveals the biological importance of ACOT8 and provides a potential combination therapy for treating patients with advanced GEM-resistant PDAC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

